Methadone is an opioid agonist therapy (OAT) that is effective for managing cravings, eliminating symptoms of withdrawal, and reducing illicit opioid use among individuals with opioid use disorders. Commonly administered in opioid treatment programs under clinic staff supervision, methadone maintenance increases retention in treatment and lowers substance use compared with nonpharmacological treatments for individuals with opioid use disorders (
1). Methadone is also a lower-cost alternative to more recently introduced OATs, including buprenorphine (
2). Treatment guidelines recommend providing access to both buprenorphine and methadone; buprenorphine has a superior safety profile, but both medications are effective in reducing opioid use and risk behaviors (
3).
Buprenorphine is now a covered benefit to some extent in every state Medicaid program, but 17 states do not include methadone maintenance therapy as a covered benefit (
4). Moreover, future Medicaid funding for methadone maintenance is being challenged in states currently providing coverage. In 2014, Maine announced a plan to eliminate methadone maintenance from its Medicaid benefit (
5). The timing of these decisions is critical, given that Medicaid expansion under the Affordable Care Act (ACA) has dramatically expanded insurance coverage for low-income adults with opioid use disorder, which creates a new opportunity for intervention.
A recent study found significant gaps in treatment capacity for opioid use disorder (
6). Despite an increase over time in the number of physicians approved to prescribe buprenorphine, the number of opioid treatment programs has not grown much. Supply of office-based prescribers and of opioid treatment programs is insufficient to meet current need for treatment. Moreover, prior research suggests that Medicaid coverage for buprenorphine increases its availability in physician practices (
7), but the relationship between Medicaid coverage of methadone maintenance and OAT use has not been studied. Medicaid coverage may be an important facilitator of OAT utilization in populations receiving public-sector opioid addiction treatment that would otherwise have access only to nonmedication therapies (such as counseling or group therapy). Specifically, if a state includes methadone in its Medicaid benefit, that treatment is available to all Medicaid enrollees, whereas public funding for OAT from nonfederal sources (including city departments of health) may not be reliably available (
8). For example, there was a dramatic decline in OAT utilization and several treatment programs closed after the Oregon Medicaid program temporarily eliminated coverage for methadone maintenance treatment (
9). Other federal sources, such as the Substance Abuse Prevention and Treatment (SAPT) block grant, also may be used to support OAT, but these funds are becoming more restricted (
10) and may support only a portion of fees, leaving the patient responsible for a significant copayment. Another reason that SAPT funding may not promote as much access as Medicaid funding is that, although Medicaid is an entitlement program that guarantees services to all eligible members, SAPT funds are only provisionally available and can be cut or restricted for budgetary reasons.
The purpose of this study was to examine differences in OAT utilization among Medicaid-enrolled adults receiving public-sector opioid use disorder treatment in states with Medicaid coverage of methadone maintenance, states with block grant funding only, and states without public coverage. This is believed to be the first study to examine the relationship between Medicaid coverage policies and use of OAT in specialty treatment among Medicaid enrollees. Specialty care is an important point of entry into treatment for individuals with opioid use disorders.
Methods
Information on states’ methadone maintenance coverage was obtained from a 2013 mail survey of Medicaid programs in all 50 states and the District of Columbia. The survey was conducted on behalf of the American Society of Addiction Medicine (
4), which categorizes states into three mutually exclusive groups: states paying for methadone maintenance in the Medicaid fee-for-service benefit, states covering it only through SAPT grants, and states with no public coverage of methadone maintenance. States where methadone maintenance was covered through both Medicaid and SAPT were not separately identified.
We linked these state-level measures to the 2012 Treatment Episode Data Set (TEDS), a database that annually includes information on 1.7 million public-sector substance abuse treatment admissions (
11). Admissions represent either new entries into treatment programs or transfers between programs. For each admission, TEDS includes information on the treatment setting, substances used, demographic characteristics, and substance use history. TEDS records whether OAT is part of the treatment plan but does not identify which medication will be administered (methadone or buprenorphine). Nationally, 98% of all individuals receiving OAT in opioid treatment programs receive methadone (versus other medications) (
12). Insurance status is an optional variable in TEDS and was reported in 36 states in 2012. We restricted our sample to Medicaid enrollees admitted to treatment for opioid use disorders (heroin or opioid analgesics) in these states. We excluded detoxification admissions, focusing on differences in use of OAT across three treatment settings: residential, intensive outpatient (three or more days per week, each with sessions of two or more hours), and nonintensive outpatient.
We calculated descriptive statistics, stratifying the sample by residence in states with no public methadone maintenance coverage, Medicaid coverage, or only SAPT coverage. We conducted t tests for pairwise differences across groups. We also estimated linear regression models for the probability that a treatment admission included OAT. The main predictor was whether an individual lived in a state with no coverage for methadone treatment, with Medicaid coverage, or with SAPT coverage only. Because a state’s coverage of OAT may influence which populations enter treatment (
9), models adjusted for individual-level demographic and substance use characteristics. We used predicted margins to calculate across states the regression-adjusted percentages of Medicaid- and privately insured individuals in OAT-based opioid use disorder treatment. Robust standard errors were used to calculate 95% confidence intervals. Analysis of publicly available secondary data was considered exempt by the Johns Hopkins School of Public Health Institutional Review Board.
Results
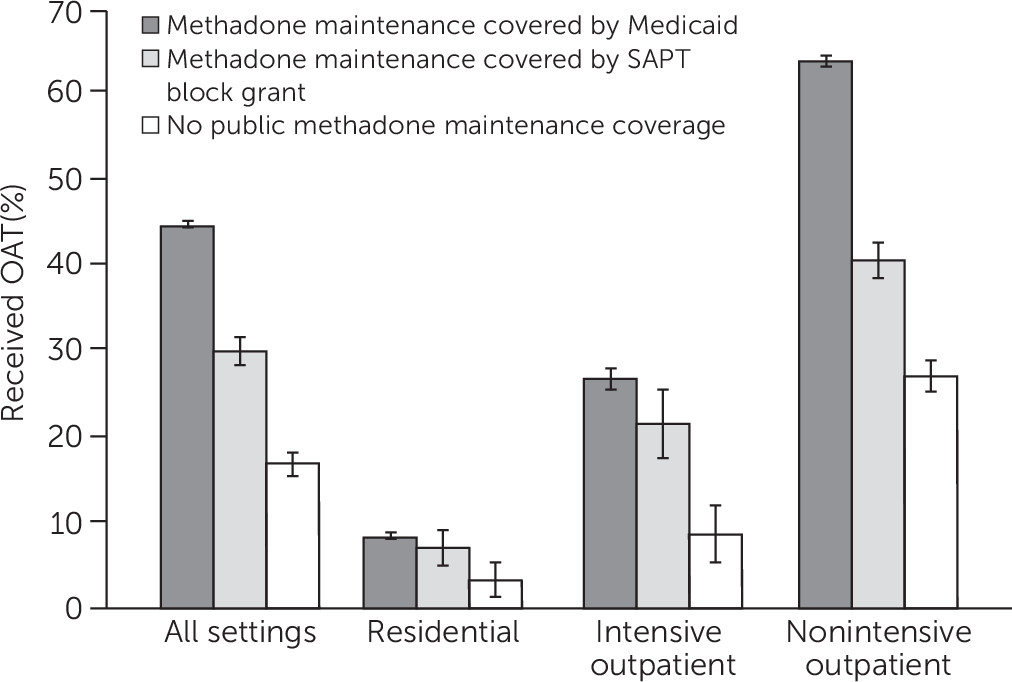
Overall, 7.0% of Medicaid enrollees in treatment for opioid use received OAT in states with no methadone coverage, whereas 46.6% received OAT in states with a Medicaid benefit that covers methadone maintenance, and 26.3% received OAT in states funding methadone only through a SAPT block grant. [Descriptive characteristics of Medicaid enrollees admitted to opioid use disorder treatment stratified by state coverage groups are reported in an online supplement to this report.]
Among enrollees in all three types of states who received any opioid use disorder treatment, nonintensive outpatient was the most common treatment setting. Females comprised the majority of Medicaid enrollees in opioid use disorder treatment in all states, especially states with no public methadone coverage. Before the ACA Medicaid expansion, most adult Medicaid enrollees were female, reflecting the program’s common eligibility categories. Most Medicaid enrollees in treatment were white, but there were large non-Hispanic black populations in states where methadone was reimbursed through a SAPT block grant. Compared with states with methadone coverage (either through Medicaid or a block grant), admissions in states with no public coverage were more likely to be individuals in a first treatment episode, to be opioid analgesic users (and much less likely to be heroin users), and to be less likely to have used substances daily before being admitted to treatment.
Figure 1 shows the differences across state groups in use of OAT after adjustment for individual-level measures of substance use and demographic characteristics. Overall, the adjusted probability of using OAT was 17.0% in states with no public coverage, 30.1% in states with SAPT only, and 45.0% in states with Medicaid coverage of methadone. Adjusted percentages of OAT use were low (<10%) in residential treatment in all three state groups. In intensive outpatient settings, the adjusted probability of using OAT was 9.0% in states without public coverage, 21.8% in states with SAPT only, and 27.0% in states with Medicaid coverage for methadone. In the nonintensive outpatient setting, where most individuals received opioid treatment, the adjusted probability of using OAT was 64.2% in states with Medicaid methadone maintenance coverage, 36.8 percentage points higher than in states with no public coverage (27.4%), and 23.3 points higher in states with SAPT only methadone coverage (40.9%).
In a sensitivity analysis, we confirmed that differences across states existed only for individuals in Medicaid and did not apply to privately insured individuals residing in the same states who would not be directly affected by Medicaid coverage policies (online supplement).
Discussion and Conclusions
Using 2012 data on admissions to publicly funded opioid use disorder treatment, we found that OAT utilization was overall nearly 30 percentage points higher among Medicaid enrollees in states with Medicaid reimbursement for methadone compared with states with no public funding for methadone maintenance, after adjustment for individual characteristics. OAT use was also significantly higher in states covering methadone with a block grant compared with no public funding, but the relative difference was smaller. These results support the notion that although block grants may facilitate some methadone treatment, they are more constrained than Medicaid financing. Medicaid coverage of methadone treatment may help drive particular populations (including heroin users) into specialty treatment.
There are some important limitations of this analysis. First, although our study focused on admissions to specialty treatment providers, some Medicaid enrollees with opioid use disorder may have entered or switched to buprenorphine treatment through office-based physician practices, which would not be reflected in our study data. In general, office-based physicians provide a minority of all addiction treatment (
13), and qualified buprenorphine providers are not available in many areas (
14). However, use of office-based providers among Medicaid enrollees is likely to be greater in states where buprenorphine, but not methadone, is covered in Medicaid. Compared with the states with Medicaid-funded methadone coverage, in states with no public coverage we found that the Medicaid populations in specialty treatment were very small, which could suggest a selection effect (
online supplement). Second, the OAT measure in the TEDS does not distinguish between methadone and other OATs, but, as noted, methadone is the dominant form of OAT in specialty programs (
12). Third, because TEDS focuses on admissions, we could not evaluate differences in duration of treatment. Prior research indicates that methadone increases retention in treatment compared with treatment without medications (
1), so we would expect that a cross-sectional sample of patients in treatment would be more heavily skewed toward OAT utilizers. Finally, with cross-sectional data we could not address how state coverage decisions may be influenced by the severity of need among Medicaid enrollees in a state. Although we adjusted for variables related to individual opioid use, it would be valuable to examine how the treated population changes after states add a methadone benefit and how such coverage may affect utilization of buprenorphine in nonspecialty settings.
Our study underscores the importance of coverage of methadone maintenance in state Medicaid programs as a mechanism to facilitate OAT. It is important to emphasize the public health benefits of facilitating access to OAT: such medications are vital for improving the health of individuals with opioid use disorders and reducing the associated morbidity and mortality. Medicaid programs that offer only nonpharmacological treatments thus risk depriving populations of highly beneficial treatments and are likely to incur greater costs associated with infectious diseases, recently illustrated by the recent rise of hepatitis C infections among injection drug users in states that do not cover methadone maintenance treatment.
Before the ACA, states were increasingly using Medicaid to expand the range of available addiction treatments for low-income adults. The scope of access is substantially enlarged now that states have additional federal funding under the ACA to cover all adults with incomes below 138% of the federal poverty line, especially childless adults who have had limited coverage options. This population has an elevated burden of substance use disorders (
15). In 2014, Medicaid-expanding states such as Kentucky, West Virginia, and Arkansas experienced some of the greatest gains in Medicaid coverage. Adults in these states have an elevated prevalence of opioid use disorders, but the state Medicaid programs do not currently cover methadone maintenance. Increasing state coverage of methadone maintenance in these and other states may be an important step toward realizing public health benefits under the ACA.