In the past two decades, clinical guidelines have recommended against long-term use of benzodiazepines and related medications (zopiclone, zaleplon, and zolpidem, also referred to as Z-hypnotics), and health agencies worldwide have undertaken, with some controversy, “antibenzodiazepine” campaigns (
1–
7). However, it appears that such recommendations have not had a significant impact on the use of these drugs (
8–
13). Major concerns regarding benzodiazepine and Z-hypnotic use are related to the development of tolerance, dependence, and addiction (
14–
16). The involvement of this class of drugs in harm caused by falls and motor vehicle collisions has also been described, particularly among older adults (
17–
23). In recent discussions, some have argued that benzodiazepines should be restricted as controlled substances (
24), but such arguments have stimulated reaction pointing to the need for education and appropriate prescribing rather than imposing a blanket ban (
25). Despite the vast body of literature on benzodiazepines and Z-hypnotics, patient characteristics affecting sustained use and the relationship between long-term use and escalation to high doses have not been fully elucidated (
26,
27).
Misinformation spread by the media (
28) tends to promote an image of the person on long-term benzodiazepine therapy as an addict who will escalate to high-dose use. Although long-term use, particularly for sleep disorders and by older adults, is not advisable (
29–
31), recommendations depend on the indication for use; in fact, individuals affected by anxiety and panic disorders might benefit from longer periods of therapy (
32–
34). The definition of long-term, or sustained, use varies in the literature, from a daily supply for at least 120 days (
13) to continuous use for at least two years (
26) and up to three years (
27). Questions remain about the risk of escalating to high doses (
13), but long-term use has not been proven to be a strong predictor of escalation. A study published in 2003 concluded that there was no demonstrable correlation between long-term use of benzodiazepines and escalation to high doses: only 1.6% of 2,440 patients receiving benzodiazepine prescriptions continuously for two years escalated to a high dose (above 40 diazepam milligram equivalents [DMEs]) (
26). Another study, conducted between 2004 and 2007 and using data from Norwegian administrative prescription databases, followed benzodiazepine users for three years and concluded that only .9% of 81,945 users showed “excessive” use (more than two defined daily doses established for each drug) (
27).
Assessing risk of tolerance among individuals on sustained use of benzodiazepines is challenging, and no evidence-based guidelines on strategies to discontinue long-term use are available (
35). In addition, clinicians can be reluctant to discontinue treatment for patients who have achieved good symptom control. Patients may also be reluctant to discontinue medications they find effective. It is important to provide evidence on the risk of dose escalation among such patients and particularly among older individuals.
Our study, conducted for the entire population of a Canadian province, investigated the characteristics of individuals who were prescribed benzodiazepines (or zopiclone) continuously for at least two years and assessed the extent of dose escalation by sustained users of these medications.
Methods
From all Manitoba residents registered with the provincial health care system from April 1, 1996, to March 31, 2008, a population of incident (new) users of benzodiazepines or related drugs (zopiclone) was identified. The time frame of the study was intentionally limited to 2008 in order to eliminate data affected by the implementation of a government-initiated intervention aimed at improving prescribers’ habits, specifically targeted at psychotropic medications. No age restrictions were applied. According to validated definitions (
29), incident users were individuals without a prescription for any of the medications of interest in the year prior to receiving their first prescription. Cohort entry date was the date of first prescription (index date).
Several exclusion criteria were applied. Patients were excluded if they had less than one full year of provincial health coverage prior to the index date and less than 27 months of coverage after the index date. As in previous studies (
26), sustained use was defined as “continuous use” for at least 27 months (two years after a baseline observation of three months to establish the initial dose) from the index date. Continuous use was based on the “persistent use” definition, by which prescription refills cover at least 80% of each of the periods of observation (
36). The end of continuous treatment was defined as the earliest of the following: end of the last prescription (dispensing date plus days’ supply) before a gap of more than 30 days; a hospital episode of care lasting longer than 30 days (because medications administered in a hospital are not captured by the prescription database); the end of health coverage; death; or the end of the study (March 31, 2008).
Ethical approval was obtained from the Health Research Ethics Board of the Faculty of Medicine, University of Manitoba. The study was conducted in compliance with the Personal Health Information Act of Manitoba, and privacy-confidentiality consent was obtained from the Health Information Privacy Committee of the Government of Manitoba.
Administrative data addressing benzodiazepine and Z-hypnotic use between April 1, 1996, and March 31, 2008, were accessed from the Manitoba Population Health Research Data Repository at the Manitoba Centre for Health Policy. Patient records within the repository are deidentified by using an encrypted personal health information number to protect privacy. Databases accessed included the Population Registry (PR), which contains demographic information of all residents of Manitoba, and the Drug Product Information Network (DPIN), which contains prescription data. DPIN captures prescriptions dispensed in Manitoba regardless of the type of coverage (government sponsored, private, or out of pocket), thus providing a comprehensive description of nonhospital drug use. No information is captured on medications administered in hospitals and on medications provided as physician samples. Prescriptions dispensed to First Nations patients served by northern nursing stations may be underestimated. However, it has been determined that the database captures over 90% of all prescriptions dispensed in the community (
37). The PR and DPIN databases were linked to Hospital Abstracts and Medical Services databases, which provide information on all encounters with the health care system for all residents of the province and facilitate assessment of individual diagnoses based on
ICD-9-CM and
ICD-10-CA diagnostic codes.
All benzodiazepines available on the Canadian market at the time of study were included. All drug identification numbers were retrieved from Health Canada drug product database by using the respective codes of the Anatomical Therapeutical Chemical classification system (
Table 1). It is important to note that reported Z-hypnotic use was limited to zopiclone, because zaleplon was withdrawn from the market in 2007 and had negligible utilization in Manitoba over the study period, as previously reported (
12); zolpidem did not become available on the Canadian market until the end of 2011.
Cohort characteristics were described, and stratifications by sex, age (0–44, 45–64, and ≥65 years), residence (urban versus rural), and income quintile were conducted. Income quintile was estimated on the basis of Statistics Canada median income of the neighborhood of residence according to the patient’s postal code. Individuals for whom neighborhood income could not be assigned (that is, residents of personal care homes, psychiatric facilities, or prisons or wards of the Public Trustee and Child and Family Services) were grouped into a “not found” category. Baseline diagnoses and other medications prescribed for each patient were also reported. A comparison of a younger group (<65 years) to an older group (≥65) was performed in response to specific concerns regarding the inappropriate use of benzodiazepines in older populations. Additional parameter assessments included years of continuous use, numbers of prescribers and pharmacies per user, and number of different agents used during the continuous-use period.
Doses were calculated, regardless of type of benzodiazepine prescribed, on the basis of DMEs per day as per the scale reported in
Table 1. The baseline dosage was determined by calculating the average monthly dose for the initial three-month period of continuous use. Dose escalation was defined as an increase in monthly dose from a baseline dose within the recommended range (<20 DMEs for individuals age <65 and <10 DMEs for individuals ≥65) to high doses (>40 DMEs for individuals <65 and >20 DMEs for individuals ≥65). Dosage evaluation was based on observations at six-month intervals.
Descriptive statistics were used to characterize the cohort of patients with sustained use of benzodiazepines and zopiclone. Chi square tests (categorical variables) and t tests (continuous variables) were used to compare the younger (0–64) and the older (≥65) segments of the population.
Dose escalation within the two-year time frame was evaluated by using mixed-model latent-class trajectory analysis (
38). Stepwise modeling was performed to test different numbers of groups, and the Bayesian information criteria values were compared to determine the model with the best fit. Multinomial logistic regression models were used for trajectory group membership. Groups with distinct trajectories were identified on the basis of our outcome of interest (dose escalation) assessed at six-month intervals. Chi square tests (for categorical variables) and F tests (for continuous variables) were used to compare trajectory groups. Analyses were performed with SAS statistical software, version 9.4.
Results
Between April 1, 1996, and March 31, 2008, a total of 6,838,899 prescriptions for benzodiazepines and Z-hypnotics were dispensed to 331,461 users in Manitoba (population approximately 1,200,000). From the cohort of incident users (individuals who had not received any prescription for any of the medications of interest in the year prior to cohort entry) and after applying all exclusion criteria, we identified 12,598 individuals as sustained users (80% yearly persistence for at least two years after the incident prescription). Data from individuals not meeting the “sustained use” definition were also analyzed: less than 7% of those received doses higher than 30 DMEs, and only 3.1% exceeded 40 DMEs.
Characteristics of sustained users are reported in
Table 2. Stratification by three age groups, prompted by previous information on benzodiazepine use in Manitoba (
12), was possible. Mean years of continuous use was 4.4 (range of two to ten years). Mean age at baseline was 57.3 years. Most users lived in urban areas (61.1%), and approximately 50% belonged to the lowest income quintiles. The most prominent diagnosis was depression (43.1%). Zopiclone was the most prescribed agent (33.3%). More than 60% of the younger patients were prescribed concomitant antidepressants; opioids were the second most common concomitant medications (34.7%), followed by antipsychotics (20.4%).
The possibility of “doctor shopping” and “pharmacy hopping” was assessed by examining prescribers per user and pharmacies per user, respectively (
Table 3). The mean number of physicians per user was 3.3 in the younger group and 2.5 in the older group. The mean number of pharmacies per user was 2.6 in the younger group and 1.7 in the older group. The differences were statistically significant.
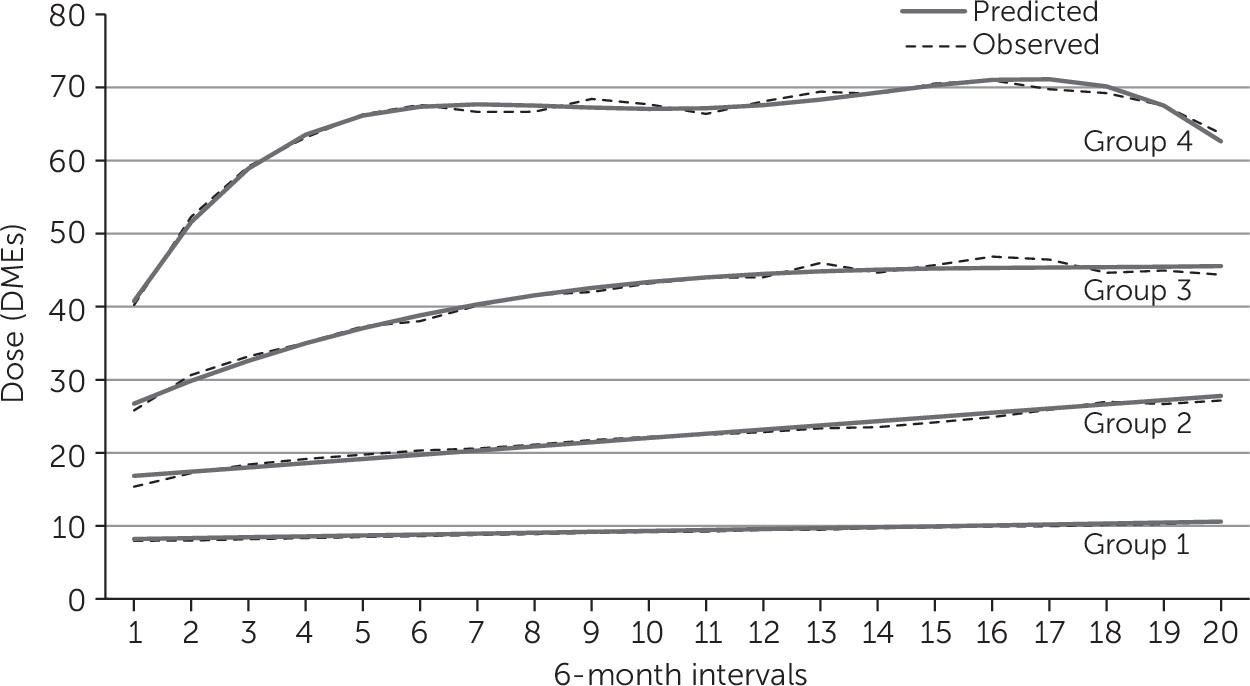
Latent-class trajectory models identified four distinct groups (
Figure 1). Group 1 included 74% of the cohort, with 9,209 individuals who started on a low initial dose (baseline value of 8.0 DMEs [observation 1]) with a final observation of 10.6 DMEs (predicted values of 8.2, 95% confidence interval [CI]=8.1–8.3, to 10.6, CI=10.4–10.8). Group 2 included 18% of the cohort and showed an increase from 15.4 to 27.2 DMEs at the end of observations (predicted values of 16.8, CI=16.7–17.0, to 27.8, CI=27.5–28.1). The average DME for group 3, which accounted for 6% of the cohort, increased from 25.8 to 44.4 (predicted values of 26.7 DMEs, CI=26.3–27.1, to 45.6, CI=44.4–46.7). Only 211 individuals—less than 2% of the cohort and less than .1% of the entire population of individuals filling benzodiazepine prescriptions in Manitoba—constituted group 4, characterized by a high initial dose (average DME of 40.2) with a dose escalation up to 63.7 DMEs at the end of observations (predicted values 40.8, CI=39.9–41.6, to 62.6, CI=60.8–64.7).
Individuals age 65 or older represented 9.6% of group 3 and 3.3% of group 4 (
Table 4). In the high-dose escalator groups, more than 55% of patients were age 44 or younger, 50% were males, and approximately 76% lived in urban areas. Short-acting benzodiazepines and zopiclone were the most prescribed in group 1 (48.2% and 38.5%, respectively) and group 2 (29.4% and 25.2%, respectively). In contrast, short-acting benzodiazepines and zopiclone were less prescribed in group 3 (17.1% and 7.4%, respectively) and in group 4 (5.7% and 0%, respectively). Clonazepam was prescribed to 6.2% of group 1 participants, 35.7% of group 2, 67.0% of group 3, and 91.0% of group 4. Outliers with “extreme” use of benzodiazepines (initial DMEs higher than 115) were excluded because of uncertainty in data reliability; these data were not used in the assessment of escalation.
Sensitivity analyses were performed for a cohort obtained after all individuals with a diagnosis of seizure disorder were excluded (data not shown). Escalation profiles were similar: groups 1 and 2 represented the vast majority of the cohort (73% and 19%, respectively), and doses remained lower than 30 DMEs for these groups. Groups 3 and 4 combined represented 8% of the cohort; doses escalated from 27.5 DMEs to 45.6 in group 3 and from 42.4 to 70.0 DMEs, in group 4. Clonazepam was the most prescribed agent in the high-dose-escalator groups (71% and 93% in group 3 and 4, respectively).
Discussion
Benzodiazepines and zopiclone are widely prescribed in Manitoba, but only 4% of benzodiazepine users fit the definition for sustained use (
26), and only .3% escalated to a dose exceeding the 40-DME level.
Our approach used a latent-class trajectory analysis that resulted in a clear definition of four groups of patients with predictable patterns in dose escalation. This statistical method has the advantage of allowing for the description of dose changes over time without predetermined adjustments that might introduce selection bias. Characteristics of groups identified by a strict and simple definition (in our case, dose escalation) can provide important hypothesis-generating information on indicators that can be used for further hypothesis-testing studies. Groups 1 and 2 had doses within the guidelines. In group 3, patients increased their initial dose slightly over 40 DMEs; this group represented 6% of the cohort of sustained users. Only group 4, which represented 1.7% of the cohort, was started at doses higher than recommended and escalated to very high doses (above 60 DMEs). Results are consistent with the conclusions of two previous reports showing that only a small segment of a population that received benzodiazepine prescriptions could be classified as sustained users and that an even smaller number escalated to doses higher than those recommended by product monographs and clinical guidelines (
26,
27).
Prescription of benzodiazepines to elderly populations has decreased (
12), and users older than 65 on long-term therapy with benzodiazepines are generally treated with lower doses. In our study, concomitant opioid use was lower in the elderly population compared with younger patients; nevertheless, the relatively high proportion of older individuals with dementia (15.7%) who were on long-term benzodiazepine treatment is reason for concern.
Benzodiazepines appear to be prescribed extensively to patients with diagnoses of depression and anxiety disorder across all age groups. Use of higher doses for such patients could indicate greater symptom severity; however, potential for misuse or abuse cannot be ruled out.
Doctor shopping and pharmacy hopping did not appear to be a major concern but were more common in the group with higher dose escalation (group 4). Even though zopiclone was the most prescribed agent, it was not demonstrably involved in significant dose escalation (only 7% of individuals received high-dose prescriptions), whereas clonazepam was the most commonly used drug among high-dose escalators (91.0% of group 4).
Strengths of our study are the comprehensive nature of the databases used. The DPIN database captures more than 90% of prescriptions filled in community pharmacies across the province, regardless of insurance coverage for prescription medications. Furthermore, benzodiazepines and other psychotropic drugs have been historically covered as unrestricted benefits by the provincial drug programs, thus eliminating issues of differential access. In addition, our large population-based study provided a long follow-up period (up to ten years), which allowed for a robust analysis of dosage changes.
Our study had some recognized limitations. Administrative data are records of medication dispensation, not of actual consumption, and no information was available on severity of illness, clinical benefits, quality of life, or lifestyle characteristics. Further, the lack of clinical data prevented the investigation of situations in which patients might have escalated to threshold levels and were not prescribed higher doses despite not having obtained—or having lost—initial clinical benefit. These patients might remain on continuous benzodiazepine therapy because of difficulties and fear of discontinuation. DPIN does not include medications received in hospitals or information on diagnoses and comorbidities; however, linkage with medical claims databases and hospital discharge abstracts allowed identification of diagnoses for each individual.
Benzodiazepines can offer some advantages over other psychotropic medications. After decades of widespread use, benzodiazepines have a well-established record of safety. They do not seem to be associated with any long-term organ damage, nor do they seem to cause significant adverse events related to weight gain, metabolic or sexual dysfunction, or movement disorders. Sustained efficacy and safety of benzodiazepines and Z-hypnotics have been demonstrated in clinical trials, and psychiatrists have reported benzodiazepines to be useful for some patients (
25,
39). Doubts have also been raised about the superiority of antidepressants over benzodiazepines in conditions such as generalized anxiety disorder and social anxiety disorder (
40,
41). Nevertheless, for patients with a primary diagnosis of depression, antidepressants should minimize the concomitant symptoms of overlapping anxiety. Concerns regarding benzodiazepine dependence and abuse should not be dismissed or underestimated, particularly for patients with a comorbid substance use disorder and for those receiving other potentially addictive agents (for example, opioids). Clinicians are faced with the challenge of identifying individuals at high risk of potentially dangerous dose escalation. Among sustained users of benzodiazepines, younger age, urban residence, low income, a diagnosis of depression, and concomitant use of antidepressants seem to contribute to dose escalation.
Conclusions
Most sustained users of benzodiazepines maintained relatively stable doses that did not exceed recommendations. Further efforts should be devoted to better understanding the characteristics of patients who receive high doses in intermittent and short-term patterns (
42); prescribers should always consider strategies aimed at mitigating harm caused by medication abuse.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy, University of Manitoba, for use of data contained in the Population Health Research Data Repository under project 2009-020 and derived from data provided by Manitoba Health.