Major depressive disorder is one of the most common mental disorders in the United States and is associated with poor quality of life, increased health care resource utilization, and high personal and societal costs (
1–
3). In 2010, major depressive disorder accounted for 3.8% (95% uncertainty interval=3.0%–4.7%) of the total 2.5 billion global disability-adjusted life years (
4). A recent study reported an economic burden of $210.5 billion in 2010, with 48% to 50% of these costs attributable to the workplace (absenteeism and presenteeism), 45% to 47% to direct medical costs, and 5% to suicide (
5).
Depression treatment has been only partially effective. Typically, in clinical trials (six to eight weeks’ duration), less than 50% of patients respond to initial treatment, and among the responders, only 50% to 65% achieve remission (
6). Patients who do not tolerate treatment or who do not show sufficient response to the initial treatment often require secondary treatment. Patients who do not respond after one or more treatment trials add substantially to the costs of depression (
7).
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) project was designed to evaluate the relative efficacy and tolerability of various treatments for outpatients with nonpsychotic major depressive disorder who do not respond to initial treatment with citalopram, a selective serotonin reuptake inhibitor (SSRI), or subsequent treatments. Patient enrollment was done between years 2001 and 2004, and the study ended in 2006. In this trial, the decision to move patients to the next level or the follow-up phase depended on clinical judgment of remission, response, and intolerance and was determined at each treatment visit. Remission was indicated by a score of ≤5 on the Quick Inventory of Depressive Symptomatology–Clinician Rating (QIDS-C16), and response was indicated by a ≥50% reduction in baseline QIDS-C16 score and a QIDS-C16 score of >5. Intolerance of medication was indicated if a participant discontinued treatment within the first four weeks for any reason or because of intolerable side effects after that time. In level 2, the decision to switch from citalopram or to augment citalopram was based on patient preference. A particular clinical implication of that decision was that the process for switching required tapering of citalopram. In STAR*D, 47% of patients responded and 33% remitted after a maximum of 14 weeks of treatment with citalopram, and 35% of the patients moved to the next level (level 2) for a secondary treatment (
8).
During level 2 of the trial, patients could receive bupropion, sertraline, or venlafaxine. Previously published results showed that the response and remission rates were not significantly different among the three drug switch options (
9). However, the cost-effectiveness of treatment among these switch options has not been evaluated. Cost-effectiveness analyses may aid the decision-making process of choosing a switch option after initial treatment failure (
10). Although there have been studies evaluating efficacy and tolerability of antidepressants, cost-effectiveness data comparing the treatments remain limited. Most of the existing economic evaluations of depression treatment focus on the first-line treatment options, and only some consider switching, titration, or augmentation in their calculations (
11). Thus decision guidelines for second-line treatment that incorporate costs remain sparse.
This study aimed to estimate the costs incurred during level 2 of the STAR*D trial and to determine whether one of the switch options was more cost-effective than the others.
Methods
The STAR*D trial design and protocol have been previously described in detail (
9,
12). STAR*D was a multilevel trial, and the patients could move to the next level or a 12-month naturalistic follow-up phase (wherein they continued the indicated treatment) depending on their therapeutic response and side effects. In level 2, 727 patients were randomly assigned to one of the drug switch options (bupropion, sertraline, or venlafaxine) for depression treatment.
Calculated costs were based on three components: antidepressant medications, other medications, and all health care facility utilization. The antidepressants included the assigned study medication. Other medications included antidepressants other than the assigned treatment that the patient might have used during level 2 and concomitant medications required to manage side effects of antidepressants. The concomitant medications included trazodone, anxiolytics, sedatives, laxatives and antiemetics, and sexual dysfunction medications. Utilization of all medication types was based on total dose intake, which depended on the specific dose and duration of use of each medication. For antidepressant medications, doses were obtained from the trial data. For concomitant medications, doses were not entered in the study and were determined by using the middle value of the ranges recommended in the UpToDate database (
13). The duration of medication use was determined by using the recorded start and end dates. [The algorithm used to determine the recorded start and end dates is available as an
online supplement to this article.]
Health care facility utilization included outpatient visits, emergency room (ER) visits, and hospitalizations that occurred during level 2. Health care facility utilization data for the course of level 2 were collected by using an interactive voice response (IVR) system. The IVR system was implemented by telephone and involved a script of recorded instructions followed by a set of questions. The patients responded to the questions by pressing the appropriate number on their phone keypad. The outpatient visits after baseline of level 2 (as specified by the protocol) were also considered. The product of the determined utilization and unit costs estimated the costs associated with health care facility utilization.
The total costs for each treatment were calculated as the sum of costs of study medication, other antidepressants, concomitant medications, and health care facility utilization during level 2. All medication unit costs were obtained from
RED BOOK (
14), and health care facility costs were obtained from the Physician Fee Schedule (
15) or the Healthcare Cost and Utilization Project (
16) databases. [A table listing the means and standard deviations of unit costs along with the information source is available in the
online supplement.] To reflect the variability and skewed nature of cost data obtained from these national databases, they were modeled by using a gamma distribution (
17,
18). All costs in the study were discounted at a 3% rate to year 2014.
All missing cost information for health care facility utilization was handled by using multiple imputation methods. The missingness of IVR data was not significantly different among the three treatment arms; we therefore considered it reasonable to assume the missing-at-random mechanism for our analyses. The Markov chain Monte Carlo method (
19) was used to impute missing patient baseline information. Also, no assumptions were made about the distribution of costs in our study sample, and nonparametric methods were used. A nonparametric propensity score multiple imputation method was used to impute missing IVR cost information (
20). This method generates a propensity score to indicate the probability of IVR missingness. The observations are then grouped based on these propensity scores, and Bayesian bootstrap imputation is applied within each group. Existing IVR cost information and patient characteristics were used to impute missing IVR cost data. Costs were compared among the groups by using Kruskal-Wallis tests. If the costs among groups were significantly different, pairwise comparisons were made by using the Bonferonni adjustment. The p values for the adjustment were calculated by multiplying the number of comparisons (N=3) with the raw p value.
Response and remission were used as measures of treatment effectiveness in this study. Remission was defined as a total score of ≤5 on the QIDS–Self-Report (QIDS-SR-16) at study exit. Response was defined as a reduction of ≥50% on the QIDS-SR-16 at level-2 exit compared with scores at level-2 baseline.
The cost-effectiveness analyses were carried out from the perspective of government as the payer. Costs included the medication costs, outpatient and ER visit costs, and costs for bed days due to hospitalizations. No indirect costs were included. The cost-effectiveness analyses were conducted by using the net health benefits (NHBs) framework (
21). The NHBs were calculated by using the following formula:
In this formula, µ
Ei and µ
Ci are the average effectiveness and costs, respectively, for treatment (i), and λ is the amount of money for which there is a willingness to pay per unit of effectiveness (“willingness-to-pay”). The treatment option with the highest NHB is considered to be the cost-effective option. For our analyses, we assumed the willingness-to-pay to be $30,000 for remission or response over the duration of level 2. Linear regression models were utilized to estimate the effect of treatment on NHBs (
22,
23). We did not adjust for the time spent during level 2 because there was no significant difference in the treatment duration among the switch therapies (
9). Further, a sensitivity analysis varied the willingness-to-pay from $10,000 to $50,000 per effectiveness outcome (remission or response). Stochastic analysis was performed by using a bootstrapping method, which involved resampling costs and effects 1,000 times randomly with replacement (in a joint manner) to obtain estimates of NHBs variability for each treatment option. The bootstrapped sample was used to determine estimates of the average NHB and percentile interval of NHBs (2.5%–97.5%) in the study.
Results
In level 2 of STAR*D, 727 patients were randomly assigned to a switch drug treatment after initial unsuccessful treatment with citalopram; 239 (33%) were randomly assigned to bupropion, 238 (33%) to sertraline, and 250 (34%) to venlafaxine. Overall, the average time (mean±SD) in level 2 was 8.9±5.03 weeks. At the end of level 2, 227 (31%) patients moved to level 3, 257 (35%) entered the follow-up phase, and 243 (33%) terminated from the study. The time spent in level 2 and in the subsequent actions was not significantly different among treatments [see online supplement].
Cost of Study Medications
The average dose prescribed for bupropion, sertraline, and venlafaxine in the respective treatment arms was 223±61 mg per day, 94±34 mg per day, and 122±53 mg per day. Over the duration of level 2, patients in the respective treatment arms used a mean total quantity of 16,308±11,711 mg of bupropion, 7,608±5,178 mg of sertraline, and 10,757±8,057 mg of venlafaxine. Accounting for medication dose intake and unit costs, the analyses showed that study medication costs were significantly different among the three treatment groups (
Table 1). The bupropion and sertraline costs were not significantly different from each other, but venlafaxine costs were significantly higher than costs for each of the other two medications (p≤.001 [Bonferroni adjusted]).
Cost of Other Medications
Besides using the assigned study medication, 694 (95%) patients used other antidepressants during level 2. All of these patients had records of citalopram use (level-1 medication) in level 2. These patients used citalopram for an average of nine days, and the average prescribed dose was 38 mg per day. Over the entire duration, patients used an average of 237±874 mg of citalopram (184±228 mg, 266±1,256 mg, and 264±831 mg by patients assigned to bupropion, sertraline, and venlafaxine, respectively). In addition to using citalopram, two patients in the sertraline group used venlafaxine, and one used bupropion. These three were because of protocol deviation or nonadherence in level 2. Costs due to use of other antidepressants were not significantly different among the three groups (
Table 1).
A total of 317 (44%) patients required at least one concomitant medication to manage the side effects of antidepressants. Among them, 124 (17%) patients used trazodone; 120 (17%), sedatives; 86 (12%), anxiolytics; 35 (5%), gastrointestinal medications; and 47 (7%), medications for erectile dysfunction. The proportion of patients requiring concomitant medications and the corresponding costs incurred were not significantly different among the three treatment options (
Table 1). Overall, the total costs related to other medications were not significantly different among the three assigned treatment groups.
Cost of Health Care Facility Utilization
Only 229 (32%) participants had complete information on health care facility utilization collected by the IVR system at level-2 exit (N=70, bupropion group; N=76, sertraline; and N=83, venlafaxine). Patients reported health care facility utilization for depression (N=140), mental health conditions (N=29), and general medical conditions (N=90). Among the 229 IVR questionnaire responders, 163 (22%) had outpatient visits (N=51, bupropion group; N=57, sertraline; and N=55, venlafaxine); 34 (5%) required an ER visit (N=12, bupropion group; N=13, sertraline; and N=9, venlafaxine), and 11 (2%) indicated hospitalization during level 2 (N=2, bupropion group; N=4, sertraline; and N=5, venlafaxine).
Table 2 compares the characteristics of patients who completed the IVR questionnaire and those who did not. The patients who had missing IVR data were more likely to have terminated from the trial, to have had shorter duration in level 2, to have had greater severity of depression at level-2 baseline, and to have exited level 1 because of intolerance. For IVR cost imputation, the propensity score model was based on the assigned treatment, achievement of the remission and response outcome, and characteristics that were significantly different between patients with or without IVR data at p≤.20.
After imputation of missing costs, the average health care facility utilization costs were not significantly different among the three groups ($1,116, $1,280, and $1,076 for patients in the bupropion, sertraline, and venlafaxine groups, respectively). The costs related to health care facility utilization recorded by the IVR system and to outpatient visits that were part of the study protocol were not significantly different (
Table 1).
Total Costs and Effectiveness
The average total costs were significantly different among groups, with venlafaxine tending to have higher costs (
Table 1). However, after adjustment for multiplicity by using the Bonferroni correction, the pairwise comparisons showed no significant differences between average total costs for the drugs. There were no significant differences in total costs among the IVR questionnaire completers in the three groups. The proportions of patients achieving remission and response for bupropion, sertraline, and venlafaxine were not significantly different (
Table 1).
Cost-Effectiveness
Table 3 shows the NHBs obtained if patients used bupropion, sertraline, or venlafaxine as opposed to spending the money in a marginally cost-effective treatment for the given willingness-to-pay of $30,000 per unit effectiveness. The average NHB represents the added advantage (in terms of health) that can be achieved by investing resources in a given treatment compared with a marginally cost-effective program that is acceptable in terms of costs and effects for a fixed willingness-to-pay. The point estimates show that when effectiveness was expressed in terms of response, venlafaxine had a numerically higher NHB compared with other drugs. In other words, the number of patients who would respond to treatment would increase by ∼20% if the costs associated with venlafaxine were spent instead on a marginally effective treatment that costs $30,000 to achieve response. When effectiveness was assessed in terms of remission, venlafaxine had the lowest NHB compared with bupropion and sertraline.
However, linear regression models showed no significant differences in NHBs among treatments for either effectiveness measure. The bootstrap percentile intervals also indicated that none of these differences were statistically significant. [A scatter plot demonstrating the uncertainty in costs and effects of the three medications is available in the online supplement.]
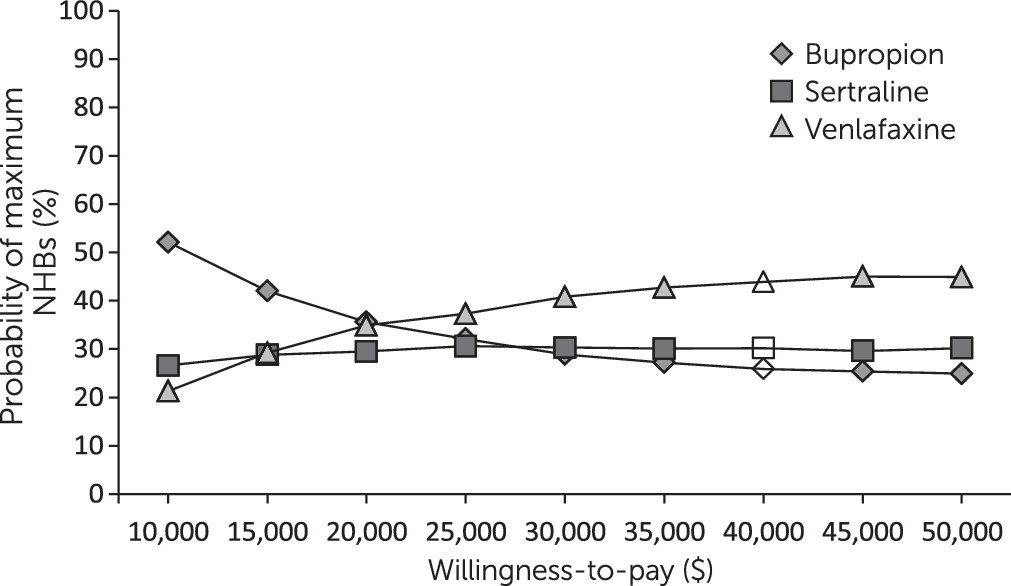
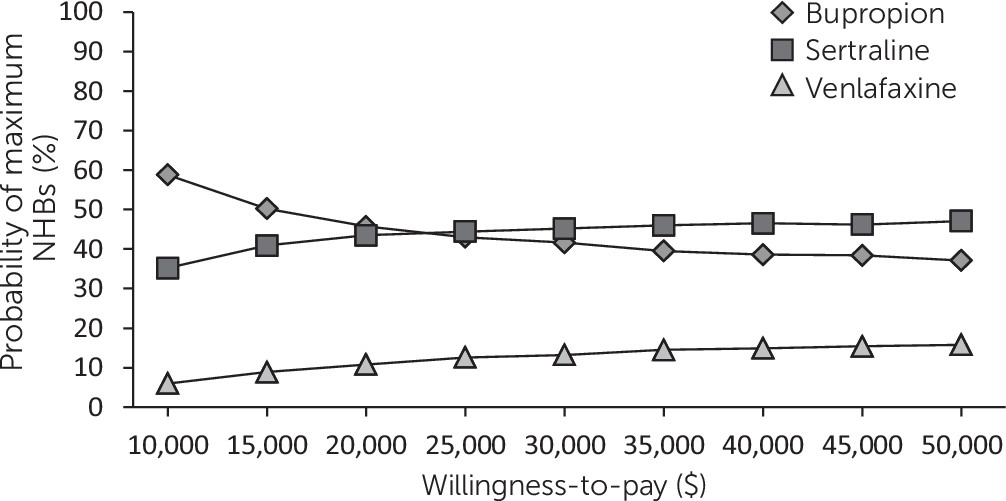
Figures 1 and
2 illustrate the cost-effectiveness acceptability curves for each medication, which show the probability of the specified treatment to have maximum NHBs compared with other treatments for a range of willingness-to-pay values. The cost-effectiveness acceptability curves for the three drugs overlapped when effectiveness was measured in terms of response (
Figure 1). When effectiveness was assessed as remission, venlafaxine appeared to be less cost-effective than the other drugs because of its slightly higher costs and because a lower proportion of patients achieved remission (
Figure 2), but the percentile intervals for the NHBs overlapped (
Table 3).
Discussion
This study demonstrated that the switch options of bupropion, sertraline, and venlafaxine in level 2 of the STAR*D project were not significantly different in terms of cost-effectiveness. The total costs among the three groups were significantly different; however, none of the pairwise differences in overall costs for the three drugs attained statistical significance. This lack of significance can be partially attributed to the large variability in costs. This study reemphasizes that overall treatment costs need to be considered rather than drug costs alone in order to estimate the costs incurred or cost-effectiveness within a clinical trial.
The literature on economic analyses of second-line treatment options for depression is sparse. Two cost analysis studies used administrative databases to compare second-line therapies for major depressive disorder (
24,
25). However, these were observational studies with biased medication prescribing patterns. In contrast, STAR*D was a clinical trial in which patients were assigned randomly to one of the investigated medications during level 2.
Two other studies used computational models to evaluate second-line treatment options for major depressive disorder (
26,
27). However, neither used generalizable cost estimates. In our analyses, we used cost estimates from national databases, such as the Healthcare Utilization Project (
16) and the Physician Fee Schedule (
15). To the best of our knowledge, no previous study has simultaneously compared the cost-effectiveness of the three switch options (bupropion-SR, sertraline, and venlafaxine-XR) after initial SSRI treatment while appropriately representing the uncertainty of the estimates.
Our study had some limitations. The health care services utilization information was collected by the IVR system and resulted in missing IVR data for 68.5% of participants. The IVR system has been associated with greater patient convenience, fewer transcription errors, and lower costs compared with personal interviews (
28). In the STAR*D trial, the IVR responses had an intraclass correlation of .68 compared with actual medical records (
29). We employed multiple imputations to minimize bias due to missingness and to appropriately represent the uncertainty of the estimates (
30). The high variance of costs in our study reflects the uncertainty in the estimated costs. Also, our analyses focused on cost-effectiveness from the perspective of government as a payer of direct costs and only during level 2 of the STAR*D study. A different perspective may yield different conclusions. For example, an individual patient could be more concerned about his or her out-of-pocket expenses than about the overall costs incurred, when considered jointly with effectiveness. Willingness-to-pay is another factor of uncertainty in our analyses; there are no recommendations for using this measure when assessing effectiveness in terms of response and remission (
31). To overcome this limitation, we systematically varied willingness-to-pay over a wide range of values on the basis of a discussion with researchers and experts. Overall, STAR*D included a broad population and was designed to closely reflect real practice (
12); thus the cost-effectiveness results of this study are highly generalizable.
Conclusions
Our results show that costs of the study medications differed significantly. However, there were no significant differences in the pairwise comparisons of total costs and cost-effectiveness of the three medications. Thus we concluded that after considering costs and cost-effectiveness, there is no rationale to change the conclusions that are based on therapeutic effectiveness. Other factors, such as clinicians’ preference, family history, or treatment of most evident cardinal symptoms, could lead to a choice of one antidepressant over another. Also, given the high uncertainty and variability associated with costs and effects, it is only appropriate to consider them together by using a cost-effectiveness framework.