The Business Case for Expanded Clozapine Utilization
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Model Design
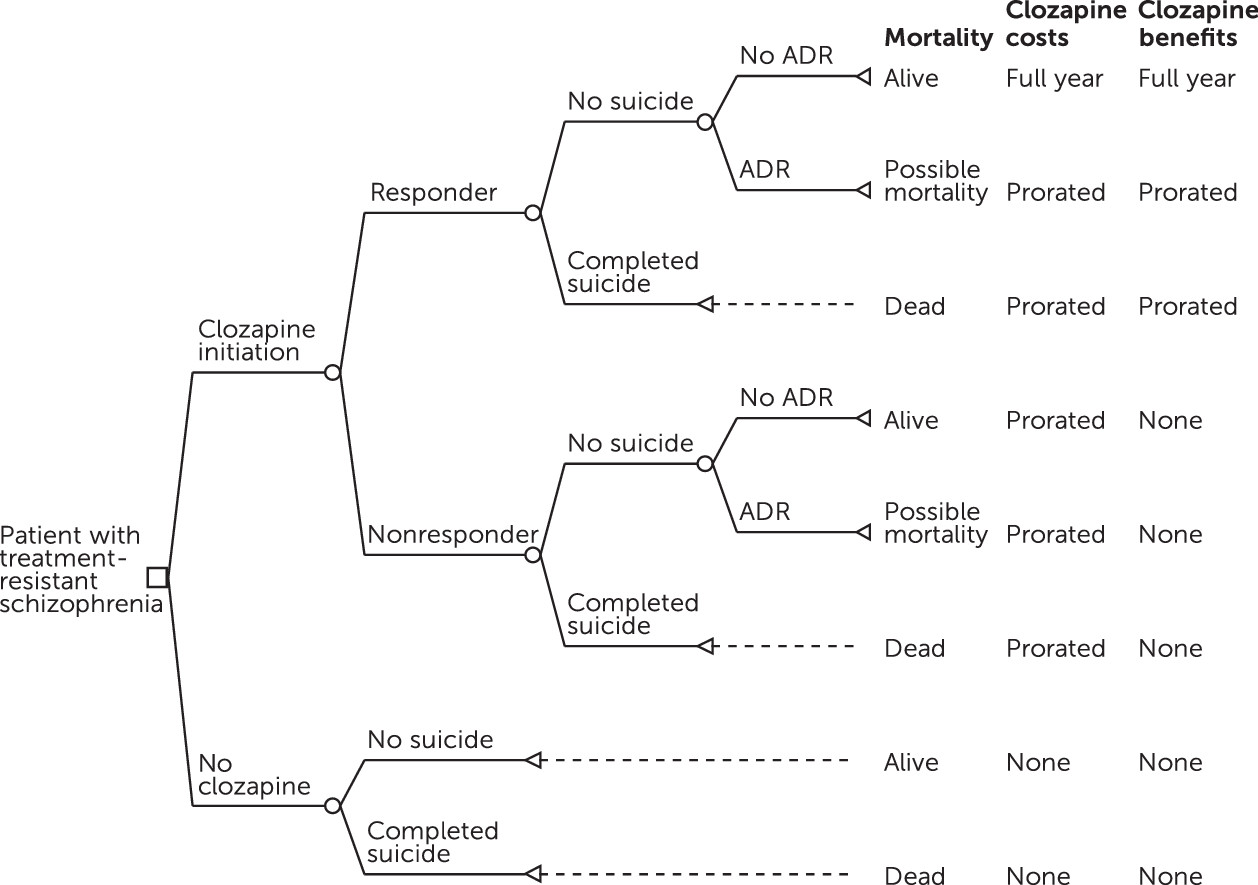
Model Inputs
Response to clozapine.
Suicide rate.
Clozapine-related ADRs.
Costs of treatment.
Costs of ADRs.
Inpatient psychiatric stays and costs.
Treatment-eligible population.
Cost Reporting
Analysis
| Variable | Base case | SD | Distribution | Notes |
|---|---|---|---|---|
| Veterans with treatment-resistant schizophrenia | 18,000 | Uniform | 2009 VHA estimate of ∼90,000 patients with schizophrenia × 20% rate of treatment-resistant schizophrenia (13) | |
| Proportion initiated clozapine treatment | .20 | Uniform | Assumption | |
| Probability of clozapine response | .510 | .01 | β | Pooled meta-analysis of 31 studies (4,19–45,47–49) [see |
| Probability of completed suicideb | .003 | β | U.S. suicide rate for ages 35–64 × SMR for suicides among persons with schizophrenia × HR for suicides among male patients with schizophrenia (57–60) | |
| Completed suicide rate given clozapine response | .34 | .8 | Log normal | Prevention of suicides with clozapine (61)c |
| Probability of ADR (clozapine arm only) | ||||
| Agranulocytosis | .005 | .0002 | β | Calculated from Cohen et al., 2012 (63) |
| Myocarditis | .0001 | .000 | β | Calculated from Cohen et al., 2012 (63) |
| Ileus | .004 | .0004 | β | Calculated from Cohen et al., 2012 (63) |
| Seizures | .032 | .003 | β | Calculated from Cohen et al., 2012 (63) |
| Diabetic ketoacidosis | .0001 | .000 | β | Calculated from Cohen et al., 2012 (63) |
| Probability of discontinuation of clozapine due to seizures | .352 | .046 | β | Calculated from Pacia and Devinsky (64) |
| Probability of mortality due to ADR | .28 | .28 | β | Calculated from Cohen et al., 2012 (63) |
| Myocarditis | ||||
| Agranulocytosis | .03 | .007 | β | Calculated from Cohen et al., 2012 (63) |
| Ileus | .20 | .036 | β | Calculated from Cohen et al., 2012 (63) |
| Diabetic ketoacidosis | .03 | .04 | β | Calculated from Cohen et al., 2012 (63) |
| Fixed costs ($)d | ||||
| Lab monitoring (event) | 10.58 | Uniform | WBC; CPT G0306 (CMS 2015 Lab Diagnostic Fee Schedule, global, nonfacility) (64) | |
| Psychiatrist visit | 43.98 | Uniform | CPT 99212 (CMS 2015 Physician Fee Schedule, global, nonfacility) (64) | |
| Veteran travel (event) | 20.00 | Uniform | Assumption | |
| Clozapine treatment (day) | 2.46 | Uniform | 100-mg tablet, 600 mg/day (VHA VISN 1 costs) | |
| Inpatient stay (day) | 1,413.70 | Uniform | 2009 VHA estimate (2015 $) | |
| Cost for ADR (2015 $) | ||||
| Seizures (year) | 1,621.62 | 245.75 | γ | MEPS 2002–2011 for adults (ICD-9 code 345) (66) |
| Myocarditis (year) | 3,695.89 | 934.70 | γ | MEPS 2002–2011 for adults (ICD-9 code 422) (66) |
| Agranulocytosis (year) | 2,428.31 | 1,641.52 | γ | MEPS 2002–2011 for adults (ICD-9 code 288) (66) |
| Ileus (year) | 6,796.33 | 1,234.79 | γ | MEPS 2002–2011 for adults >18 (ICD-9 code 560) (66) |
| Diabetic ketoacidosis (single hospitalization) | 20,141.34 | 20,415.43 | γ | (67) |
| Day of suicide | 183 | Uniform | Assumption | |
| Day of discontinuation of clozapine | 183 | Uniform | Assumption | |
| Day of ADR (clozapine arm) | 183 | Uniform | Assumption | |
| Inpatient days | 138 | Poisson | Pooled analysis of 7 studies (33,34,36,38,39,68,69) [see | |
| Reduction in inpatient days among clozapine responders | –37e | Poisson | Unstandardized mean difference in inpatient days of 7 studies (33,34,36,38,39,68,69) [see |
Results
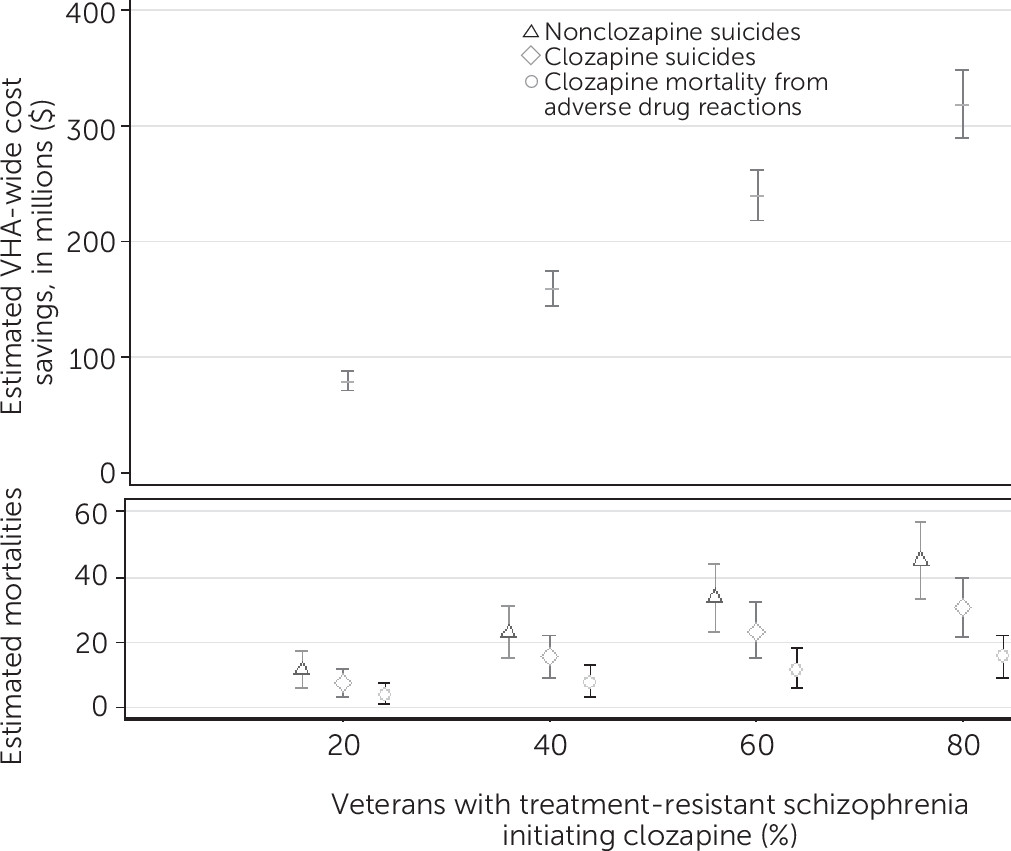
Cost Savings
| Outcome | Base case | Simulated outcome (percentile) | ||
|---|---|---|---|---|
| Mean | 5th | 95th | ||
| Aggregate cost and utilization | ||||
| Cost ($) | ||||
| Control arm | 700,410,000 | 695,541,000 | 633,082,000 | 757,851,000 |
| Clozapine arm | 619,613,000 | 615,697,000 | 560,652,000 | 671,316,000 |
| Cost savings ($) | 80,797,000 | 79,845,000 | 72,406,000 | 87,685,000 |
| Inpatient days | ||||
| Control arm | 495,000 | 492,000 | 447,920 | 536,000 |
| Clozapine arm | 429,000 | 426,000 | 388,000 | 464,000 |
| Inpatient days saved | 67,000 | 66,000 | 60,000 | 72,000 |
| Clozapine responders | 1,787 | 1,794 | 1,629 | 1,964 |
| Adverse drug reaction (ADR)b | ||||
| Seizures | 116 | 115 | 94 | 137 |
| Agranulocytosis | 16 | 16 | 10 | 23 |
| Myocarditis | <1 | <1 | 0 | 1 |
| Ileus | 16 | 16 | 9 | 23 |
| Diabetic ketoacidosis | <1 | <1 | 0 | 2 |
| Mortality | ||||
| Suicides (control arm) | 11 | 11 | 6 | 17 |
| Suicides (clozapine arm) | 8 | 8 | 3 | 12 |
| Deaths due to ADRs | ||||
| Agranulocytosis | <1 | <1 | 0 | 2 |
| Myocarditis | <1 | <1 | 0 | 1 |
| Ileus | 3 | 3 | 1 | 6 |
| Diabetic ketoacidosis | <1 | <1 | 0 | 1 |
ADRs
Deaths
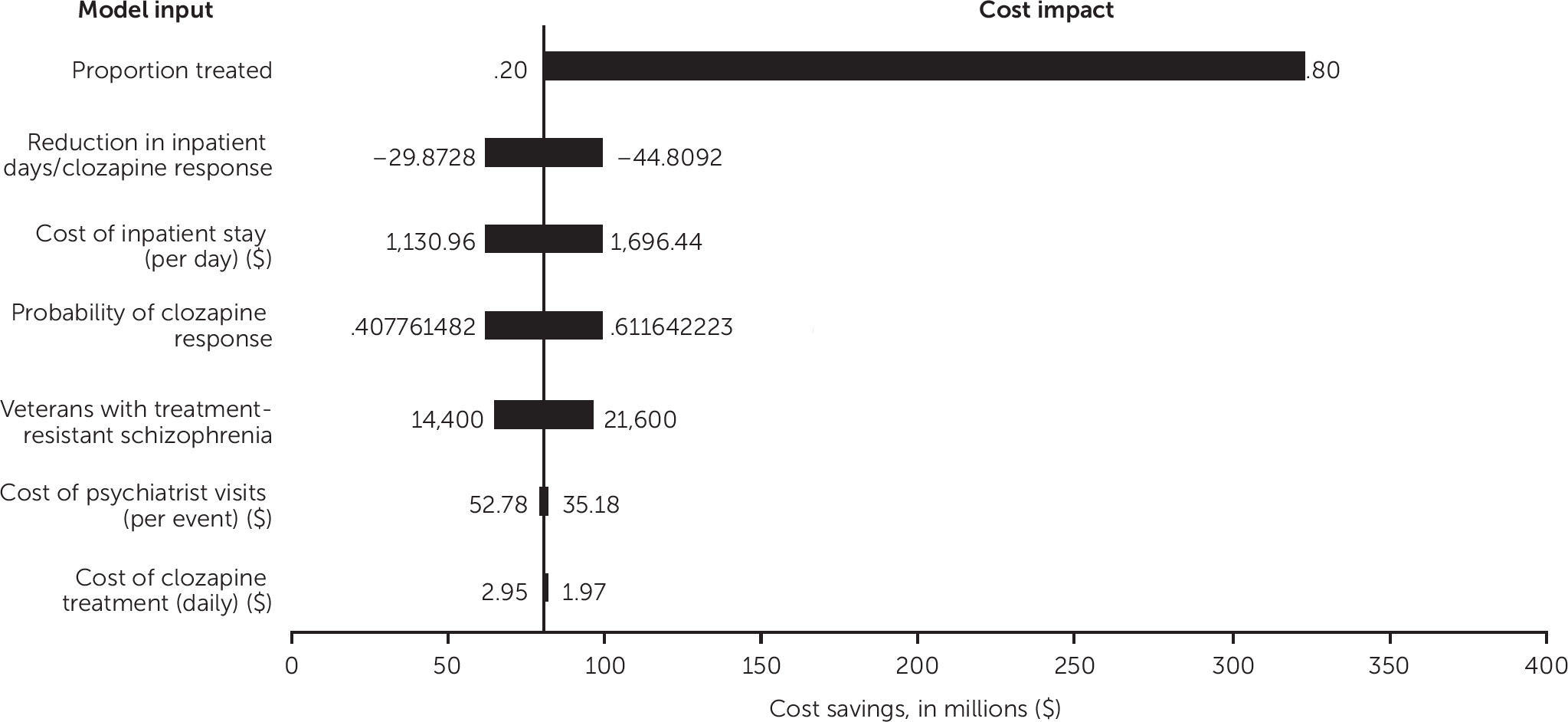
Sensitivity Analysis
Discussion
Conclusions
Footnote
Supplementary Material
- View/Download
- 72.50 KB
References
Information & Authors
Information
Published In

Cover: covered jar with star decoration, by Solomon Grimm, 1822. Glazed red earthenware. Gift of Ralph Esmerian. American Folk Art Museum, New York City. Photo: John Begelow Taylor; American Folk Art Musuem/Art Resource, New York City.
History
Authors
Competing Interests
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing PsychiatryOnline@psych.org or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
