The rate of second-generation antipsychotic prescribing for U.S. pediatric populations has increased significantly (
1,
2). Studies have documented a substantial rise both for indications approved by the Food and Drug Administration (FDA) and for off-label indications (
3). Children in foster care, juvenile justice programs, and Medicaid are particularly likely to receive a second-generation antipsychotic (
4,
5). Causes are thought to be multifactorial and synergistic (
4,
6).
Up to 20% of U.S. youths meet criteria for a psychiatric disorder, and the health care burden for this population is tremendous (
4). Childhood psychiatric illnesses cause substantial morbidity and mortality, and the annual estimated cost exceeds $240 billion dollars (
7). Access to child psychiatrists is frequently limited, as is availability of nonpharmacologic treatment resources, such as behavioral therapies and parent skills training. Provision of care often falls on primary care providers, but many pediatric physicians report insufficient time and training to assess and manage complex psychiatric complaints. Thus there is a strong need for quick, efficacious, and cost-effective treatment options that are widely available. Second-generation antipsychotics, introduced in the 1990s, are generally perceived to be safer than first-generation antipsychotics and have a demonstrated ability to treat a variety of psychiatric symptoms. This perceived safety and utility likely contributed to their rapid rise in popularity.
The FDA has approved various second-generation antipsychotics for treatment of pediatric bipolar disorder, schizophrenia, and irritability associated with autism spectrum disorder. These drugs are frequently used to treat aggression, disruptive behavior, attention-deficit hyperactivity disorder (ADHD), posttraumatic stress disorder, anxiety, tics, and insomnia and are employed as augmentation agents for treatment of depression. In fact, most antipsychotic prescriptions for individuals under age 18 are for off-label indications (
4). This pattern of high-frequency non-FDA–approved prescribing raises concerns. Evidence-based support for off-label efficacy is limited, particularly regarding long-term use (
2). Also worrisome is the well-recognized potential for cardiometabolic side effects, including weight gain, insulin resistance, and hyperlipidemia. All second-generation antipsychotics carry a black box warning about their potential to cause diabetes mellitus, and some studies suggest that young people may be more vulnerable than adults to this adverse effect.
Multiple epidemiologic studies have identified subgroups of youths who are more likely to receive second-generation antipsychotics. Medicaid-enrolled children, for example, are prescribed these medications at roughly twice the rate of children with private insurance, and rates for those in foster care are estimated to be five times higher still (
8,
9). Therefore, policy makers prompted Medicaid agencies to exercise greater control over pediatric antipsychotic prescribing. States responded by developing oversight programs, and as of August 2014, 31 states had created prior-authorization (PA) programs, which vary greatly in scope (
10). These programs, although labor intensive and costly, are commonly used because of their ability to decrease prescribing of certain medications, with associated pharmaceutical cost savings. However, relatively little is known about the effects of PA programs targeting second-generation antipsychotics. The few studies that have examined the issue among adults were highly heterogeneous and yielded mixed results (
11–
14). Some suggested the possibility of negative outcomes for specific populations, including treatment discontinuation and subsequent use of high-intensity, high-cost services. Very few studies have examined the potential for compensatory prescribing—that is, substitution of a drug from an alternative psychotropic class for the restricted drug.
Even less is known about the impact of PA policies on prescribing patterns for children. One recent study by Stein and colleagues (
15) compared Medicaid pharmacy data from two mid-Atlantic states over four years. One state used a PA program for children up to age 12. The authors concluded that the PA program resulted in a modest but statistically significant drop in prescribing for children ages six to12 but not for younger children. Constantine and colleagues (
16) determined that a PA program in Florida for children under age six was effective in decreasing the prescribing rate for second-generation antipsychotics. To our knowledge, no studies have examined the impact of a second-generation antipsychotic PA policy on prescribing for youths older than 12 or on compensatory prescribing for children of any age.
In 2011, West Virginia Medicaid implemented a PA program for second-generation antipsychotic use by very young children (under age six), which was expanded in August 2015 to include patients under age 18. An effort was made to ensure that prescribers conformed to best-practice recommendations, such as those of the American Academy of Child and Adolescent Psychiatry. Treating clinicians were required to submit data on patients’ body mass index (BMI), fasting lipids, and fasting blood glucose (or HbA1c) and to formally assess patients for abnormal involuntary movements at baseline and prior to continuation. If blood work was unobtainable because of patient noncooperation, the requirement was waived. If a second-generation antipsychotic was prescribed at an FDA-approved dosage for an FDA-indicated diagnosis, then it was approved for one year. If prescribed for an off-label use or outside the recommended dose range, then the PA was initially denied and the prescribing clinician was required to submit a letter of appeal. Appeals were reviewed by a board-certified child and adolescent psychiatrist who determined approval status and duration.
The primary objective of this study was to assess the impact of the expanded PA program on second-generation antipsychotic prescribing for pediatric patients ages two to 17. We hypothesized that after program implementation, there would be an overall decrease in the percentage of Medicaid-enrolled children receiving second-generation antipsychotics. A secondary goal was to examine whether the PA requirement led to compensatory prescribing of drugs from alternative psychotropic classes. Final objectives were to examine the differential impact, if any, of the PA program on prescribing rates by the age of the child and for individual antipsychotic drugs.
Methods
During the study period (September 2014 to April 2016), West Virginia Medicaid offered coverage to children under three programs: a traditional Medicaid fee-for-service (FFS) arrangement, the Children’s Health Insurance Program (CHIP), and several managed care organizations. According to U.S. Census Bureau statistics, approximately 55% of West Virginia’s pediatric population is covered under one of these Medicaid programs (
17,
18). We used a retrospective, interrupted time-series (ITS) design incorporating deidentified Medicaid FFS and CHIP administrative claims data. Claims data were collected on September 1, 2016, and were examined from September 2014 to April 2016. Monthly data were extracted for nearly 200,000 Medicaid enrollees ages two to 17 years and for multiple psychotropic drug classes (N=273,369 prescriptions). Specifically, drug classes of interest were first- and second-generation antipsychotics, stimulants and other ADHD medications, antidepressants, anticonvulsants, lithium, benzodiazepines, and nonbenzodiazepine hypnotics. The number of enrollees per month receiving psychotropic prescriptions was examined for each drug and each class. The total number of prescriptions for each drug and class was also recorded. During the study months, no other psychotropic drug class carried a categorical PA requirement. [A list of medications within each drug class is available in an
online supplement to this article.]
Unfortunately, the prescriber specialty was not reported consistently, and the prescribing indication or diagnosis was not always apparent. For example, we were unable to separate anticonvulsants prescribed for mood stabilization from those prescribed for other indications, such as epilepsy and migraine. Certain demographic information was unavailable, such as sex and race, because it was withheld from claims data for deidentification purposes.
To evaluate prescribing while taking into account the change in population over the study period, prescribing rate was defined as the percentage of total Medicaid and CHIP enrollees each month receiving one or more prescriptions for the drug or drug class of interest. This measure also had the advantage of avoiding “double counting” individuals who received multiple prescriptions per month within a single class.
The study was approved by the Marshall University Institutional Review Board.
Statistical Analysis
The data were analyzed in SPSS version 22 by using an ITS design, via an autoregressive integrated moving average (ARIMA) (1,0,0) model. An ITS design was chosen for its ability to account for natural trends in the data beyond just the policy change being studied and because this design is considered one of the better tools to study the effect of policy change on population-level data (
19). An ARIMA was used to account for autocorrelation in the data. Data were analyzed by using linear segmented regression and plotted over time, with the Y axis modeling the prescribing rate (defined above). Our regression can be written as Y=(baseline trend)×t
1+C+(trend change)×t
2+(level change)×phase, where Y is the prescribing rate predicted by the model, t is an integer representing time in months (1, 2, 3, and so forth), and C is the intercept. The factor t
1 is the number of months since the start of the trial, and t
2 is the number of months since the PA program began. In months before the PA program, t
2 is set to 0. Level change is a constant representing the change in intercept—in other words, the immediate change in prescribing rate after implementation of the PA program. Phase is a Boolean variable set to 0 for all months before the PA program and to 1 for all months afterward. Because t
2 and phase are 0 for all months prior to the PA program, these months can be written as Y=(baseline trend)×t
1+C. Baseline trend, trend change, and level change are reported. For ease of reading, we report the final trend (baseline trend+trend change) instead of the change in trend. Segmented regression analysis requires at least 100 observations per data point in order to achieve acceptable variability. Therefore, categories with fewer than the minimum number of observations were omitted from the analysis. The level of significance was set at p≤.05.
Results
Table 1 shows the number of enrollees prescribed a psychotropic drug and the number of prescriptions for each drug class and each month of the study. There were more prescriptions per month than individuals receiving prescriptions, because some received multiple prescriptions in a single drug class. The mean number of Medicaid enrollees per month is also provided. Monthly enrollment slowly increased over the study period and ranged from 167,008 to 190,656. Simultaneously, the prescribing trend across nearly all drug classes both before and after the PA program trended downward. The lone exception was antidepressants, which were prescribed at roughly a steady rate throughout.
Prescribing Patterns Before PA Program Implementation
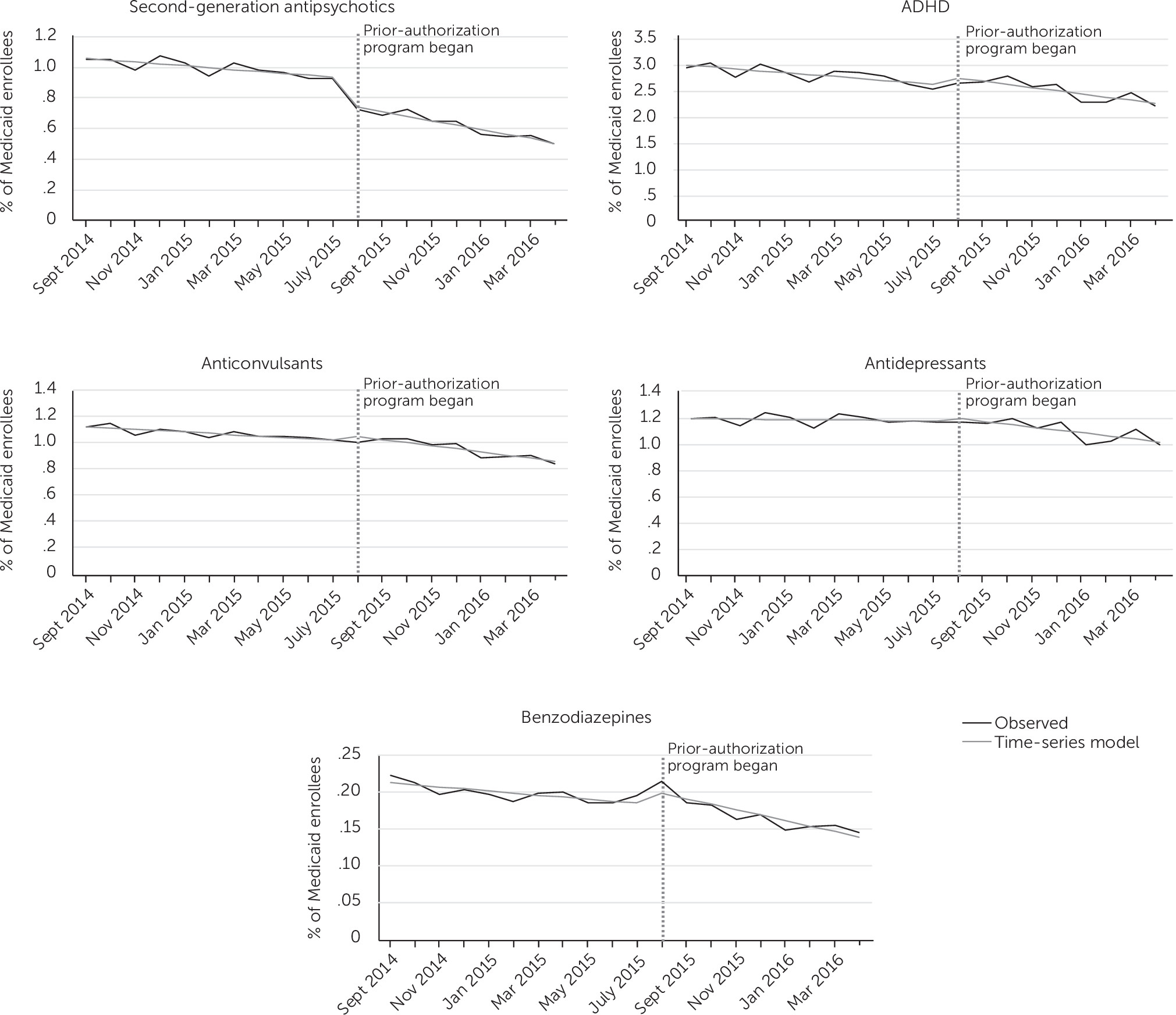
Figure 1 shows the percentage of enrollees who received a prescription per month (observed prescribing rate) over the course of the study. The rate for second-generation antipsychotics trended downward from September 2014 (1.03%) to July 2015 (.91%), the month before PA program implementation. ADHD medications were by far the most prescribed psychotropic class, followed by antidepressants, anticonvulsants, and second-generation antipsychotics. Benzodiazepines, first-generation antipsychotics, hypnotics, and lithium were all prescribed at comparatively low rates.
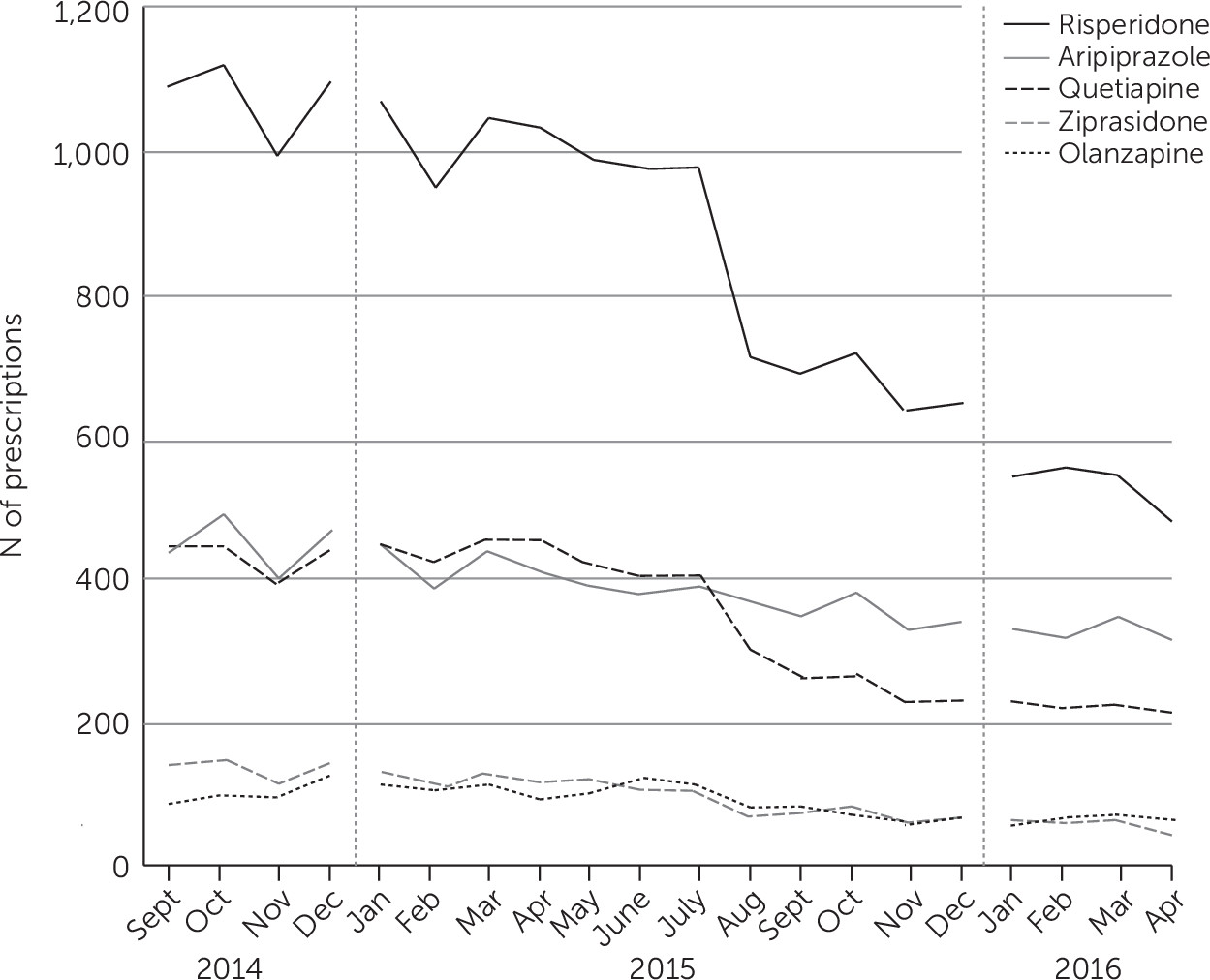
Figure 2 shows the number of prescriptions monthly for various second-generation antipsychotics. Risperidone was the most frequently prescribed, followed by aripiprazole and quetiapine. Risperidone was prescribed more than twice as frequently as any other antipsychotic. When examined separately (data not shown), prescribing patterns of second-generation antipsychotic for children ages 13–17 were very similar to those for children ages seven to 12. The prescribing rate was consistently higher in the 13–17 group than in the group ages seven to 12. The rate for second-generation antipsychotics was very low for children ages six and under.
Prescribing Patterns After PA Program Implementation
The prescribing rate for second-generation antipsychotics dropped from .91% in July 2015 to .70% in August 2015, reflecting a decrease of 351 patients. This one-month drop was quite substantial because the preceding 11 months had seen a cumulative decrease of only 161 enrollees. Even when the model adjusted for the monthly fluctuation in prescribing rates, it showed an effect nearly as large, with an estimated 17% fewer enrollees (N=289) prescribed second-generation antipsychotics than predicted by the preintervention slope had there been no PA program (p<.001).
Table 2 shows the slope values for second-generation antipsychotic prescribing both before and after the PA program. After program implementation, there was a decline in the prescribing rate that persisted through the remainder of the study (p<.001). The preintervention slope indicated a decrease of approximately 21 enrollees per month. During the time after the PA program, this rate of decline was approximately twice as steep, with a decrease of 49 enrollees per month. Thus the PA requirement coincided with both an acceleration of the preprogram trend toward less antipsychotic prescribing and a one-time absolute drop in enrollees prescribed these agents that carried through to the end of the study. The decline in the rate was statistically significant for all individual second-generation antipsychotics except for aripiprazole.
Relatively few changes were noted in prescribing trends for other drug classes. The prescribing rate remained highest for stimulants and ADHD medications. Prescriptions for these medications also showed the most monthly variability, which likely reflected prescribing changes related to the school year. However, no significant change in slope was observed for these drugs. There was a one-month increase in benzodiazepine prescriptions immediately after the PA program that could have represented compensatory prescribing. However, by the second month, this number had returned to preprogram levels. In fact, when the one-month increase was excluded and the postprogram slope recalculated, there was no significant change from the preprogram prescribing trend. A small but significant decrease in the prescribing rate was seen for anticonvulsants and antidepressants after PA program implementation. For anticonvulsants, the trend was likely attributable to a significant one-month drop in April 2016, whereas the change in the postprogram slope for antidepressants was likely significant only because the preprogram slope was nearly flat.
Discussion
Consistent with prior studies, implementation of a PA program resulted in an overall decrease in prescribing of the target drug class as measured by a significant decline in the monthly percentage of individuals prescribed second-generation antipsychotics. The decline was more pronounced than that observed in previous studies. However, previous reports examined the effects of PA policies on younger children only, who are less likely than older children or adolescents to receive antipsychotics (
15,
16). Furthermore, a unique feature of the PA program studied is the required submission of metabolic data, BMI, and formal testing for abnormal involuntary movements. These requirements added to the administrative burden for both clinician and reviewer and may have resulted in fewer prescriptions. However, when policy makers weighed the need for this extra step, strong consideration was given to the vulnerability of the state’s pediatric population to these adverse effects. West Virginia has the highest rate of diabetes in the nation, with similarly poor ratings for cardiac health. Multiple studies have demonstrated relatively low rates of metabolic monitoring for patients taking second-generation antipsychotics, despite the black box warning and best-practice guidelines (
20). It would be interesting to know to what extent the PA requirement improved metabolic monitoring; however, the answer is beyond the scope of this study.
Claims data showed that after PA program implementation, the prescribing rate for every second-generation antipsychotic except for aripiprazole declined significantly. The reasons are not readily apparent, although multiple factors could explain this finding. The FDA has approved aripiprazole for multiple pediatric indications, including schizophrenia, bipolar disorder, and autism spectrum disorder. Furthermore, it is possible that aripiprazole was not prescribed as often as other drugs for off-label indications (such as ADHD and oppositional defiant disorder). If so, then aripiprazole was less likely to be denied and to require an appeal.
A PA requirement for second-generation antipsychotics may lead to “class switching,” or a compensatory increase in use of an alternative drug class when the preferred class is less easily accessible. For example, in a study of adults with schizophrenia or bipolar disorder, we might have expected a compensatory increase in prescriptions for first-generation antipsychotics or mood stabilizers, respectively. Aside from the lone increase in benzodiazepine prescribing during the first month after PA program implementation, we did not observe this pattern. However, because our data did not include the indication for each prescription, we were unable to detect small changes in the prescribing rate for anticonvulsants prescribed specifically for mood stabilization.
Most young people take second-generation antipsychotics for conditions not easily treated with pharmacotherapy. A possible result of PA program implementation is “therapy switching,” or a compensatory increase in use of alternative psychosocial therapies. Examination of claims for nonpharmacologic treatments, such as behavior therapy, play therapy, and other psychosocial interventions, may be a good direction for further research.
A potential consequence of PA requirements could be full treatment discontinuation and a subsequent need for high-intensity services, such as hospitalization (
11,
12). The relatively few studies in this area have focused on adults with schizophrenia or bipolar disorder—populations known to be at high risk of treatment noncompliance—whose illness exacerbations often lead to severe decompensation. To date, no studies have evaluated adverse or unintended outcomes of PA programs targeting children’s use of antipsychotics. This was beyond the scope of our study because data on use of alternative services were not available. However, we speculate that full treatment discontinuation is theoretically less likely for pediatric patients than for adults because of differences in the target psychopathology. Also, treatment compliance for children is typically enforced by caregivers. However, given the potentially severe consequences of treatment discontinuation, this area requires further research.
Our investigation had several other limitations. We did not use comparable data from another second state as a control. Our data represent prescribing patterns for Medicaid FFS and CHIP that may not readily generalize to Medicaid “medical management only” or private insurance. Also, prescribing patterns can vary greatly by geographic location, and our results may not be representative of Medicaid programs in other states. The number of monthly observations for first-generation antipsychotics, lithium, and hypnotics was too small to permit statistical assessment. Similarly, monthly antipsychotic prescribing for children ages zero to six was insufficient for subgroup analysis.
Conclusions
Implementation of a PA program targeting second-generation antipsychotics for children under age 18 was associated with a significant decrease in the prescribing rate for this class of medication. The decrease was not accompanied by sustained compensatory prescribing for any other psychotropic drug class. Additional research is needed to investigate the effects of PA policies on subsequent utilization of nonpharmacologic treatments and high-intensity services.