Randomized Trial of Interventions for Smoking Cessation Among Medicaid Beneficiaries With Mental Illness
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Study Sites
Participants
Procedures
Interventions
Program coordinators.
Motivational tobacco education.
Usual care prescriber visit for smoking cessation.
Cessation condition 1: usual care PV only (PV only).
Cessation condition 2: PV plus facilitated quitline counseling (PV+Q).
Cessation condition 3: PV plus CBT (PV+CBT).
Incentives for smoking abstinence.
Measures
Abstinence.
Other smoking-related measures.
Mental and general medical health measures.
Participant Flow
Statistical Analyses
Descriptive analyses.
Stratum effect.
Outcome analyses.
Safety analyses.
Results
Study Participants
| Total sample (N=661) | PV only (N=146) | PV+Q (N=303) | PV+CBT (N=212) | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % |
| Age (M±SD)b | 45.0±10.8 | 43.0±10.8 | 45.0±10.7 | 46±11.0 | ||||
| Female | 426 | 64 | 82 | 56 | 200 | 66 | 144 | 67 |
| White | 610 | 93 | 131 | 91 | 821 | 93 | 198 | 93 |
| High school graduate | 549 | 83 | 116 | 80 | 247 | 81 | 186 | 87 |
| Not employed | 545 | 82 | 113 | 77 | 258 | 85 | 174 | 81 |
| Diagnosis | ||||||||
| Schizophrenia spectrum disorder | 148 | 22 | 31 | 21 | 74 | 24 | 43 | 20 |
| Bipolar disorder | 150 | 23 | 29 | 20 | 68 | 22 | 56 | 26 |
| Major depression | 158 | 24 | 36 | 25 | 69 | 23 | 53 | 25 |
| Anxiety and other disorders | 205 | 31 | 50 | 34 | 94 | 31 | 62 | 29 |
| Modified Colorado Symptom Index score (M±SD)c | 49.1±10.9 | 49.5±11.9 | 49.0±10.8 | 48.0±10.2 | ||||
| Lifetime psychiatric hospitalizations (M±SD) | 8.3±26.9 | 6.0±15.4 | 9.0±34.7 | 9.0±19.0 | ||||
| Blood pressure category | ||||||||
| Prehypertension | 303 | 46 | 61 | 42 | 147 | 49 | 95 | 45 |
| Hypertension | 170 | 26 | 45 | 31 | 73 | 24 | 52 | 25 |
| Obese | 371 | 56 | 90 | 62 | 160 | 53 | 121 | 57 |
| Tobacco use | ||||||||
| Breath carbon monoxide (M±SD ppm)d | 25.3±16.9 | 25.0±17.3 | 27.0±16.8 | 24.0±16.8 | ||||
| Fagerström Test for Nicotine Dependence score (M±SD)e | 5.3±2.3 | 5.0±2.4 | 5.0±2.1 | 6.0±2.4 | ||||
| Cigarettes smoked per day (M±SD)b | 17.3±10.5 | 16.0±10.9 | 18.0±10.2 | 17.0±10.5 | ||||
| Quit attempt in past year | 347 | 52 | 69 | 47 | 155 | 51 | 123 | 58 |
| Confidence in quitting score (M±SD)f | 3.7±1.2 | 4.0±1.2 | 4.0±1.2 | 4.0±1.2 | ||||
Treatment Outcomes
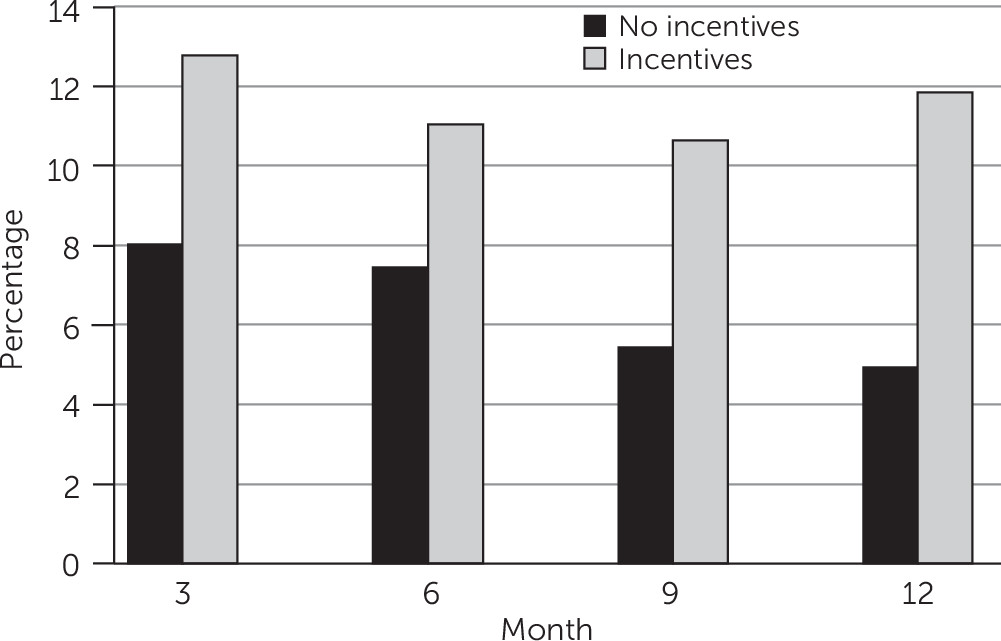
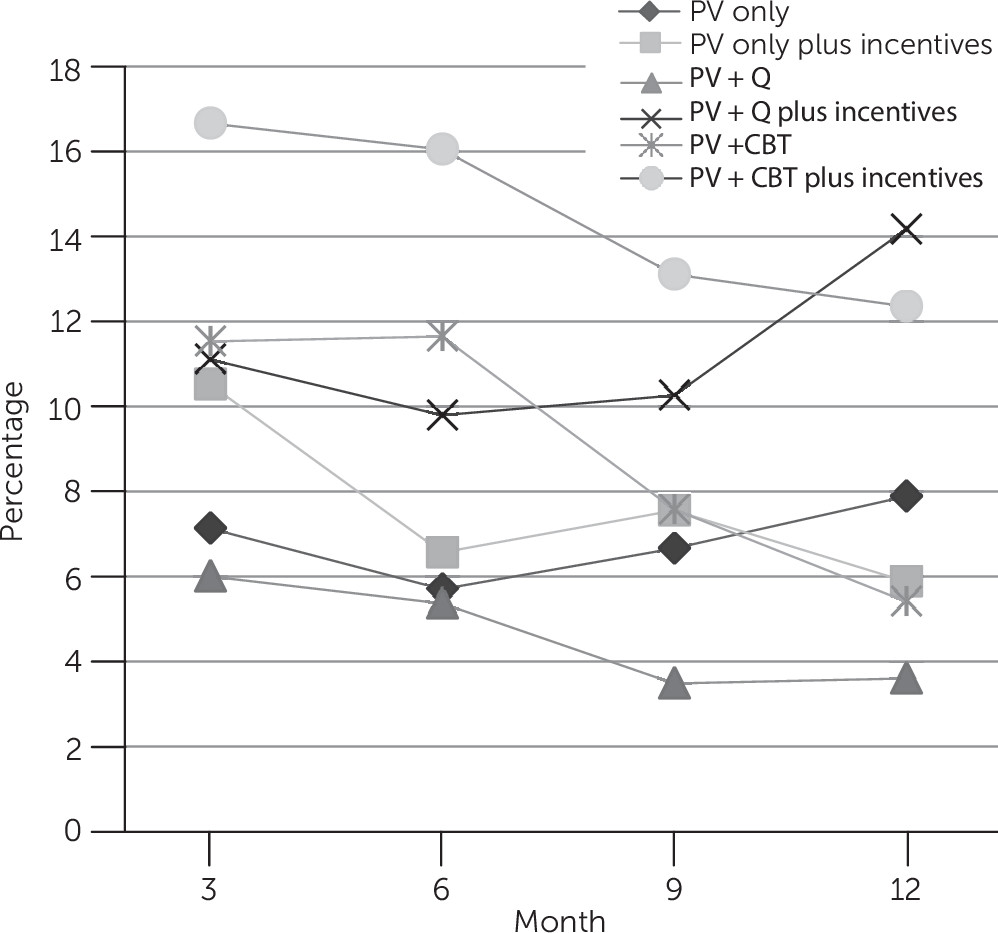
Treatment Safety
Treatment Program Participation
Discussion
Conclusions
Acknowledgments
Footnote
Supplementary Material
- Download
- 91.48 KB
References
Information & Authors
Information
Published In

Cover: Flying Geese, by Felix Bracquemond, 19th century. Black chalk, brush, and watercolor, highlighted with white gouache. Museum purchase, Davis Museum, Welleseley College. Photo credit: Davis Museum/Art Resource, New York City.
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
