The United States is dealing with a rampant opioid epidemic with far-reaching health and social consequences. The number of adults who use heroin or misuse prescription opioids has grown rapidly in recent years, along with fatal and nonfatal opioid overdoses (
1,
2). In this context, the use of opioid agonist therapy (OAT) has assumed increased relevance. There is strong evidence supporting the efficacy, effectiveness, and cost-effectiveness of treatment with methadone and buprenorphine, the only two opioid agonists approved by the U.S. Food and Drug Administration for long-term management of opioid use disorders. Yet, many individuals with opioid use disorders do not receive OAT or any substance use treatment at all (
3).
Use of OAT for treatment of opioid use disorders may have increased in recent years following expansion of Medicaid under the Affordable Care Act (ACA). Past research has found improved access to OAT in states that opted to expand Medicaid (
4–
8). However, past analyses were mainly based on drug utilization and pharmacy data, the number of providers able to prescribe OAT, or service-level data.
In this study, we used data from the Treatment Episodes Data Set–Admissions (TEDS-A) for patients who received specialty outpatient substance use treatment during the periods surrounding implementation of Medicaid expansion to assess the association between this policy and use of OAT. This study extended previous analyses of TEDS-A that covered admissions up to 2015 and examined OAT use in various settings (
9). The analyses in this study included TEDS-A data from 2010–2016; focused on ambulatory care settings, which are the main treatment settings for specialty OAT; and explicitly examined the extent to which change in OAT treatment was mediated by a larger proportion of admissions with Medicaid in expansion states.
Methods
TEDS-A has been described in detail (
10). Briefly, TEDS-A is an administrative database managed by the Substance Abuse and Mental Health Services Administration for tracking admissions to specialty substance use treatment programs receiving public funding. This data set includes information from a large majority of these substance abuse treatment facilities. However, it does not capture medical offices, where a significant proportion of buprenorphine is prescribed (
11). Programs are required to report some basic clinical and demographic information for each admission (e.g., type of substance use disorder and services). Other information (e.g., health insurance) is collected by some, but not all, states.
We limited the main analyses to TEDS-A data on 943,430 admissions to regular or intensive outpatient services in 2010–2016 for treatment of primary opioid use disorder in the 31 states that reported on health insurance or expected source of payment and use of OAT in addition to the District of Columbia and Puerto Rico. Expansion status was determined on the basis of a Kaiser Family Foundation report (
12). All states that had implemented expansion before the second quarter of 2015 were classified as “expansion states.” States that had not implemented expansion before the second quarter of 2015 were classified as “nonexpansion states.”
Medicaid insurance status was based on the patient’s health insurance and expected or actual primary source of payment for the treatment episode. To allow us to capitalize on all available data, patients were classified as having Medicaid if Medicaid was recorded as the type of health insurance or as the primary source of payment for the episode of care. In admissions with both types of data, there was a high degree of agreement between the two—Medicaid was the primary source of payment for 80.2% of admissions with Medicaid insurance.
Type of substance use disorder was recorded separately for the “primary,” “secondary,” and “tertiary” substances. We limited the analyses to admissions in which the primary substance use disorder involved “heroin,” “nonprescription methadone” or “other opiates and synthetics.” The last category included “buprenorphine, codeine, hydrocodone, hydromorphone, meperidine, morphine, opium, oxycodone, pentazocine, propoxyphene, tramadol, and any other drug with morphine-like effects.” The usual route of drug administration, including IV use, was also recorded.
Use of OAT was determined on the basis of reporting in TEDS-A that “the use of opioid medications such as methadone or buprenorphine will be part of the patient’s treatment plan.” The specific type of OAT medication used was not recorded. History of any previous substance use treatment was also recorded, but type or setting of previous treatment was not recorded.
In addition, the patient’s sex, age, race-ethnicity, and homelessness status at the time of admission were recorded.
Analyses were conducted in two stages. First, temporal trends in OAT use over the 2010–2016 period were examined in expansion and nonexpansion states. Next, difference-in-differences (DID) analyses were used to assess the association between Medicaid expansion and use of OAT. A series of logistic regression analyses with OAT use as the outcome were conducted. In model 1, the variable for period (2010–2013 versus 2014–2016) was entered to examine change in OAT provision over time. In model 2, expansion status was added to examine independent effects of time period on OAT use. Model 3 added an interaction term for period × expansion status, which represents the differential change in the use of OAT in expansion states compared with nonexpansion states following Medicaid expansion (i.e., the DID estimate). Finally, the patient’s Medicaid insurance status was added in model 4 to assess whether any differential change in the use of OAT from model 3 was mediated by an increase in the proportion of admissions with Medicaid. To quantify mediation, we compared the percentage change between model 3 and model 4 in the regression coefficient (i.e., log odds ratio) for the interaction term of period × Medicaid expansion (
13). The regression models additionally adjusted for sociodemographic characteristics, prior treatment, and IV drug use.
In further analyses, we examined whether differences in coverage of OAT under different state Medicaid programs explained the difference between expansion and nonexpansion states in OAT treatment following the implementation of Medicaid expansion. To this end, we compared expansion states in which Medicaid programs did and did not cover methadone (
14). All expansion states included in the analyses covered buprenorphine.
All analyses included a state-level random effect to account for nesting of admissions within states. All analyses were conducted by using Stata 15.1 software. The study used publicly available deidentified administrative data that are exempt from informed consent requirements and institutional review board review.
Results
Most admissions were by male patients (55.4%), patients who were non-Hispanic white (74.7%), and patients between the ages of 25 and 49 years (74.4%). About half (48.4%) reported IV use as the route of drug use, and 73.0% reported receiving prior substance use treatment. Heroin use disorder was the most common type of primary opioid use disorder (65.1%), followed by other opiates and synthetics (33.7%) and nonprescription methadone (1.2%).
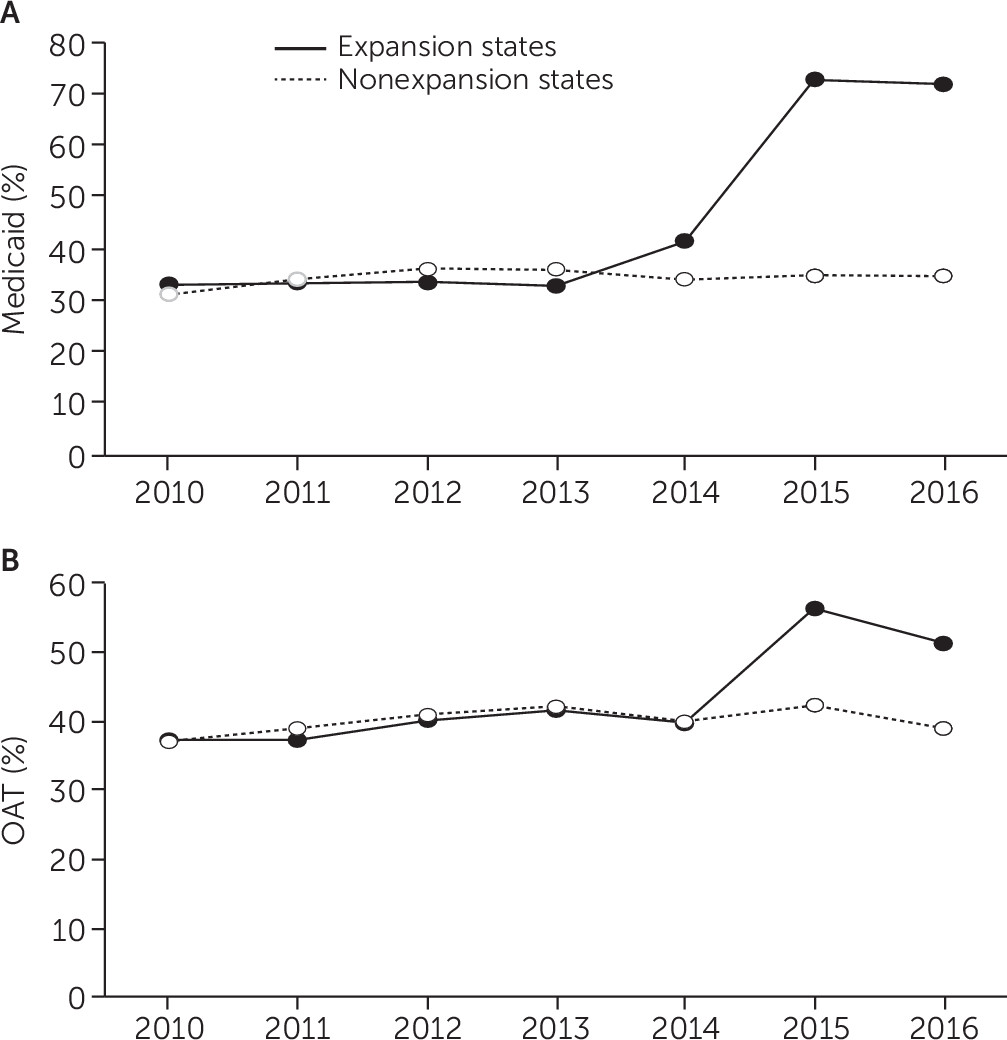
The increase in the proportion of admissions with Medicaid from 2010–2013 to 2014–2016 was much larger in expansion states than in nonexpansion states (33.3% to 64.4% versus 34.5% to 34.6%, respectively). Similarly, the increase in the proportion of admissions involving use of OAT was more pronounced in expansion states than in nonexpansion states (39.1% to 50.2% versus 39.9% to 40.5%, respectively) (
Figure 1).
In random-intercept logistic regression analyses, the odds of OAT use increased significantly between 2010–2013 and 2014–2016 (adjusted odds ratio [AOR]=1.16, 95% confidence interval [CI]=1.15–1.17) (see details for model 1 in the online supplement). Furthermore, the odds of OAT use appeared to be higher on average over the whole 2010–2016 period in expansion states than in nonexpansion states, although this difference was not statistically significant (see details for model 2 in the online supplement). The interaction term of period × expansion state was statistically significant, indicating that the increase in OAT use after implementation of Medicaid expansion was significantly larger in expansion states than in nonexpansion states (AOR=1.28, 95% CI=1.24–1.32) (see details for model 3 in the online supplement).
In model 4, the regression coefficient for time period × expansion state was greatly attenuated by the addition of Medicaid insurance and was no longer statistically significant (see online supplement). These results indicate that the effect of time period × expansion state status was mostly explained by the larger proportion of admissions with Medicaid insurance in expansion states versus nonexpansion states in 2014–2016. The interaction of time period × expansion status was also a significant predictor of Medicaid insurance (AOR=3.91, 95% CI=3.80–4.04; data not shown). Furthermore, Medicaid insurance was strongly associated with OAT use, irrespective of expansion status (AOR=1.79, 95% CI=1.77–1.81) (see details for model 4 in the online supplement), supporting the hypothesis that the increase in OAT use following expansion was mediated by the larger number of admissions with Medicaid. Approximately 90% of the differential increase in OAT use in expansion states could be explained by the greater proportion of Medicaid-covered admissions in these states.
In further analyses limited to expansion states, use of OAT increased following Medicaid expansion even in states that did not cover methadone in their Medicaid programs. Use of OAT increased from 11.7% to 20.8% of admissions in states that did not cover methadone and from 41.1% to 52.7% of admissions in states that covered methadone.
Discussion
Following implementation of Medicaid expansion, there was a larger increase in OAT use for opioid use disorders in expansion states than in nonexpansion states. This difference was primarily mediated by the larger increase in the proportion of admissions with Medicaid in expansion states than nonexpansion states. This is a welcome development, given that OAT remains the major evidence-based treatment for opioid use disorder. Despite progress, however, a substantial percentage of admitted patients with opioid use disorder in both expansion and nonexpansion states were not treated with OAT. For many adults with opioid use disorder, insurance coverage alone may not be sufficient to ensure access to evidence-based treatments. Improving access to OAT may require outreach, reorganization of treatment services, and recruitment of medically trained staff that can prescribe OAT.
Better understanding of pathways by which Medicaid expansion might lead to expanded OAT access could also provide guidance regarding strategies to further expand access. Medicaid coverage may give patients a greater choice of treatment programs and allow them to choose services affiliated with medical facilities in which OAT is more readily available. Alternatively, having a larger patient population covered by Medicaid may encourage services to offer OAT by hiring medically trained staff with prescribing privileges. It is also plausible that expansion states may have been aided in expanding their provision of OAT by the more robust coverage of OAT under their Medicaid programs even before implementation of expansion (
15). However, we found increases in the use of OAT in expansion states with and without Medicaid coverage of methadone.
In interpreting these results, limitations of TEDS-A should be considered. First, information on health insurance was not available for several states. The association between Medicaid coverage and OAT use in those states may be different. Furthermore, TEDS-A includes limited information about the surveyed facilities and does not record the programs’ size, affiliation with medical institutions, or staffing.
Conclusions
In the context of the limitations described above, the findings provide evidence that recent insurance policy reforms have contributed to increased use of OAT for management of opioid use disorder in substance abuse treatment facilities. Although Medicaid expansion and other provisions of ACA strive to improve financial access to OAT, optimal use of OAT calls for reorganization of services and health system reforms to make these treatments more readily available.