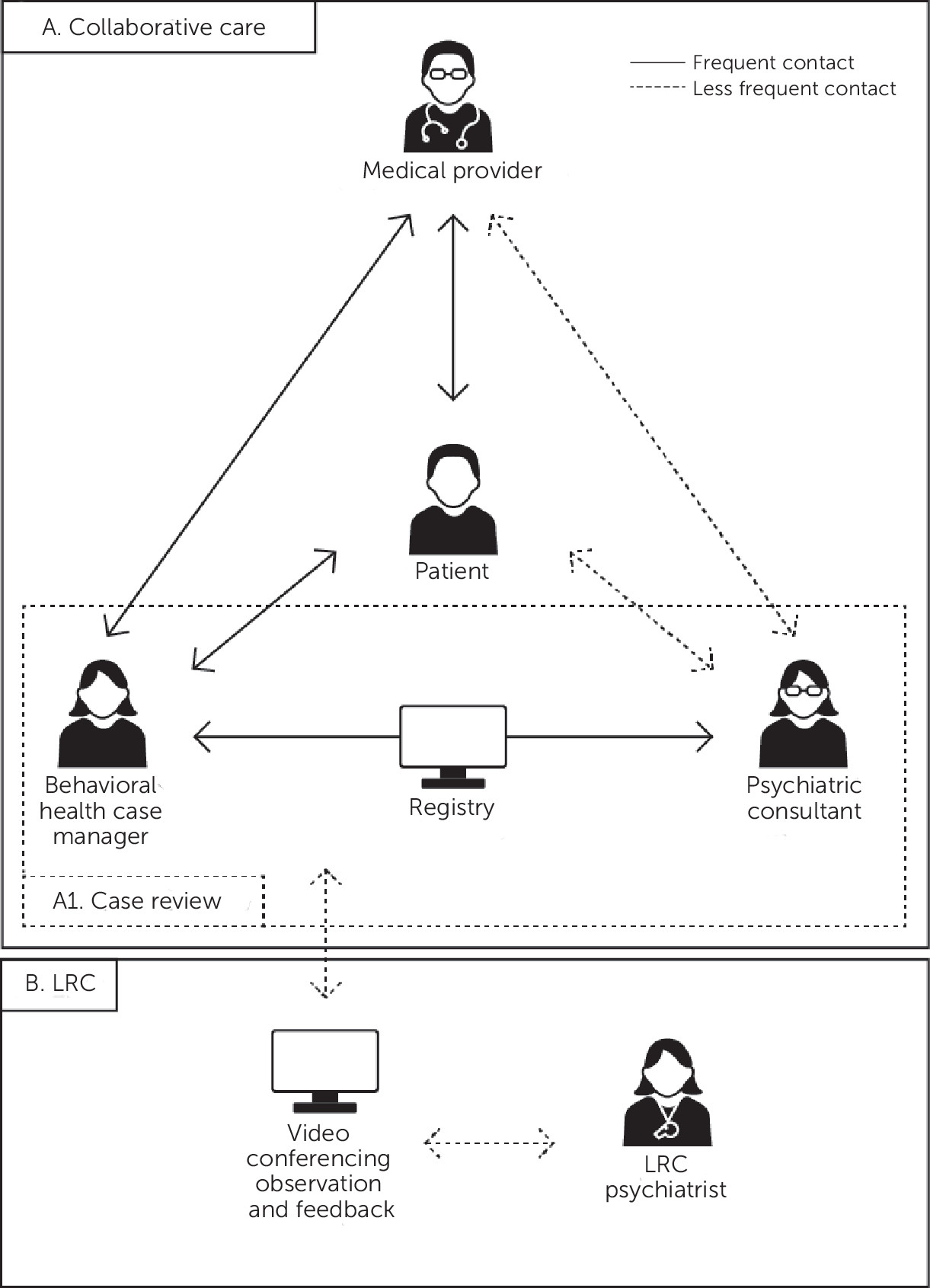
The collaborative care model (CoCM) is an integrated behavioral health framework for the treatment of common mental disorders in primary care. Strong evidence supports its efficacy and cost-effectiveness in diverse populations and in clinical settings, including prenatal care and primary care (
1,
2). CoCM is a population health model that tracks defined patient populations in registries. Symptoms are measured regularly, and treatment is actively changed until clinical goals are achieved. Trained primary care or prenatal providers along with behavioral health professionals in the clinic provide evidence-based medication or psychosocial treatments and are supported by regular systematic case review (SCR) with a psychiatric consultant (
3). During SCR, care managers and psychiatric consultants use a patient registry to guide treatment recommendations for a panel of patients.
Despite the model’s effectiveness, there are barriers to implementing CoCM with sustained fidelity because it is a complex multicomponent intervention that requires training multiple personnel and redesigning systems of care. Although training and technical assistance are rigorous, monitored, and sustained in research settings, support during implementation is often limited to a one-time training session with minimal support thereafter, reducing the likelihood of success. Identifying scalable and effective implementation strategies for CoCM is critical to accelerating the adoption of evidence-based mental health care in primary care settings.
SCR is a critical element of CoCM that allows for population- and measurement-based care and is associated with better patient outcomes. However, many psychiatrists and psychiatric trainees report low confidence in their ability to conduct SCR. We have found that key challenges to implementing SCR include a requirement to reorganize resources, a team approach that can be unfamiliar to both the primary care team and the psychiatric consultant, and lack of experience with the clinical successes of a fully functioning CoCM practice that reinforces fidelity to the model.
Practice facilitation (external facilitation or coaching) are quality-management implementation strategies that can improve uptake and help maintain fidelity in the delivery of evidence-based practices (
4). Coaches support changing practices through a range of implementation strategies, such as training, assessment of progress (audit and feedback), technical assistance, and quality improvement, carried out in an iterative manner and guided by care data (
5). Implementation of complex care processes, including both procedural and clinical elements, can require facilitation by a coach who has significant specialized experience and skill, limiting the ability to support many sites through standardized training alone. Additionally, ongoing or longitudinal coaching is more effective than one-time training for making significant and sustained changes in the behaviors of mental health clinicians that result in improved patient outcomes (
6). On the basis of these considerations and positive experiences over the course of ongoing implementation efforts (
7), we evaluated a novel longitudinal intervention-specific coaching model for CoCM teams, longitudinal remote coaching (LRC), as an implementation strategy for perinatal CoCM. LRC uses intervention-specific coaching and facilitation during SCR, a key process within CoCM during which all CoCM members are available at the same time. The intervention-specific coaching that LRC offers is distinct from but complementary to general practice facilitation, which targets more-general clinical processes. Thus, LRC addresses a gap in the implementation support system for complex interventions such as CoCM; it addresses specific needs of the generalist-specialist interaction early, at a critical developmental period of implementation to help these new teams gain the experience needed for clinical success (
5). In LRC, a psychiatrist with expertise in CoCM and perinatal CoCM coaches a local clinical team that is new to the model by regularly joining their routine SCR meetings via video conferencing to observe and provide feedback in order to support implementation of and fidelity to CoCM (
Figure 1). Direct observation is the major component of LRC, and additional activities include ongoing training in CoCM, review of fidelity measures with follow-up steps to improve fidelity, and addressing obstacles to implementation. Using remote video and audio communications technology for this observation enhances the scalability of LRC. The LRC expert evaluates individual and team functioning to assess mastery of CoCM. Next, we describe the design and feasibility of LRC for perinatal CoCM in three clinics. To our knowledge, this is the first description of LRC for a complex multicomponent intervention such as CoCM.
Development and Initial Testing of LRC
Data reported here were collected in the context of a multistate, prospective, pragmatic, cluster-randomized trial to assess LRC for perinatal depression in safety-net health centers (
clinicaltrials.gov identifier: NCT02976025). Clinic-based CoCM teams were randomly assigned to receive standard implementation support alone or with LRC. Standard implementation support involved prelaunch training and workflow development for 2–3 months, a 2-day in-person training session based on adult learning principles prior to initiation of services, and postlaunch support via regular training calls with care managers (CMs) to develop clinical skills. General practice facilitators (professional facilitators from participating organizations) also supported the formation and dynamics of the health center implementation teams.
We used expert recommendations, field testing, and iterative modification to develop a standardized process for LRC and a feedback form to provide systematic input to the teams. We then provided LRC to three clinics randomly assigned to the intervention in the clinical trial. We conducted an exploratory mixed-methods analysis of LRC data from these three clinics and of interviews with CoCM team members who received LRC.
Refining the LRC process and feedback form.
To refine the LRC process and the feedback form, we convened a group of faculty and research collaborators with expertise in CoCM and in delivering training and implementation support for CoCM teams. These stakeholders provided input on the organization of SCR meetings and on support for teams on the basis of prior research and implementations. Next, we pilot tested LRC over 4 months. Eight teams (out of 20 approached) in mature CoCM programs chosen by convenience sampling provided input, which informed the iterative modification of the tool until there were no additional significant changes. We based the tool and its refinement on established principles of CoCM (
3).
The final LRC form included key CoCM metrics, which the LRC psychiatrist obtains from the registry prior to the LRC session, including total number of patients on the caseload, number of new patients, number flagged for psychiatric case review, patients with no psychiatric case review and who showed no improvement, and number of patients with no contact with a CM in more than 8 weeks; qualitative feedback on preparation and agenda, enrollment, engagement, clinical outcomes, and team process; and information on case discussions, including reason for psychiatric review, whether new recommendations were made, and time spent discussing each case.
The standard LRC schedule was six biweekly sessions in the first 3 months, then three monthly sessions, followed by three bimonthly sessions, for a total of 12 sessions over 12 months. This schedule could be modified based on a clinic’s overall progress in meeting program metrics, achievement of skill mastery, or perceived need. For example, in keeping with the principle of graded training, in which learners who are not progressing receive additional training and support (
8), up to three additional LRC sessions could be added for a maximum of 15 sessions. After each session, the LRC psychiatrist summarized observations and answered questions from the CoCM team. The LRC psychiatrist then sent the completed standardized LRC form to the clinic within 2 business days. The clinic CoCM team could contact the LRC psychiatrist by e-mail with any questions about the form. Additionally, each LRC session began with a review of the previous session and an invitation for CoCM team members to share their thoughts or questions from the previous session.
Analysis of LRC data and participant feedback.
One of three CoCM experts (A.B., A.M.B., J.U.) joined each of the three CoCM teams for their SCR and completed a feedback form for each session attended. We calculated the percentage of sessions adherent to the recommended schedule and categorized teams as either complete (if they included both a psychiatric consultant and a CM) or not complete. Using data from standardized LRC feedback forms, we conducted a content analysis of patient case reviews and of LRC qualitative feedback in five categories (preparation and agenda, enrollment, engagement, clinical outcomes, and team process). (The Consolidated Criteria for Reporting Qualitative Research Checklist is available in the online supplement.) Content analysis involved coding and interpreting text material. For participant feedback, we used data from semistructured telephone interviews (conducted by W.E.) with CoCM team members (three CMs, two psychiatric consultants, one project lead) in a modified grounded-theory analysis, an inductive qualitative analysis technique. Our semistructured interview guide was based on the consolidated framework for implementation research and is available upon request. Interviews were recorded, transcribed, checked, and coded by W.E. and F.B. and analyzed with Nvivo.
Feasibility and Acceptability of LRC
Twenty-six LRC sessions were conducted with three clinic-based CoCM teams over 10 months. Across all three clinics, 58% (N=15) of LRC sessions occurred according to the recommended schedule. One clinic had a complete CoCM team in place for the duration of LRC implementation support. For the other two clinics, the team was complete for 0% (N=0 of six sessions) and 50% (N=4 of eight sessions with team members in attendance), respectively.
LRC content.
During the 26 observed sessions, 60 unique case discussions were conducted. Engagement issues were addressed in 30% (N=18) of discussions and diagnostic questions, in 27% (N=16). Other case discussions focused on issues such as psychosocial complexity. With respect to LRC feedback, 150 discrete recommendations were made by the LRC psychiatrist. The most common recommendation was for the clinic to use the registry regularly (25%, N=28). Other feedback was framed around core CoCM principles: population-based care (e.g., low enrollment rates, importance of relapse prevention plans); measurement-based treatment to target (e.g., prioritizing care on the basis of both severity of symptoms and improvement over time); and evidence-based care (e.g., considering all possible treatments, not only medications).
Participant feedback.
Participant feedback from independently conducted interviews with CoCM team members included a perception that LRC supported better patient care (e.g., “two heads are better than one,” LRC introduced “things I don’t think about”) and that it supported participants’ application of new skills in systems-based practice (e.g., provided an “outline of how to review cases and prioritize things”). Participants reported challenges with scheduling and with receiving the feedback forms.
Discussion
Our analysis of the LRC implementation strategy for perinatal CoCM indicated that it was well accepted by clinical teams and was perceived to support CoCM implementation. Across clinics, 58% of LRC sessions occurred according to the suggested schedule, underscoring the feasibility of the recommended frequency of LRC sessions. Clinical teams were incomplete for several LRC sessions in two of the clinics, making it challenging to comment on key care processes. Recent findings suggest that having a complete care team that includes a psychiatric consultant is important in sustaining CoCM (
9).
Analysis of qualitative content from LRC feedback forms indicated that a majority of case-related discussions focused on engagement issues. The emphasis on engagement may reflect the fact that these CoCM teams were newly formed or incomplete and therefore were more focused on building their caseloads. Although early engagement in CoCM treatment is positively correlated with positive patient outcomes (
10), other processes, such as treatment to target and instituting relapse prevention plans, are also important, and as programs matured, LRC feedback suggested increased attention to these processes.
Because incomplete care teams may preclude feedback on key CoCM processes, we have specified that clinics enter implementation only after they have identified and hired their CM and psychiatric consultant, and we have revised the LRC timeline such that it begins only after the complete CoCM team (including psychiatric consultant and CM) is in place. Future research should evaluate the relationship between LRC participation and program-level outcomes in order to develop flexible benchmarks for LRC completion.
Our analysis had limitations; it was based on a small sample, which limited our ability to assess the generalizability of our findings. Analysis of site variability regarding current level of mental health integration will be conducted upon completion of the trial. However, the results so far are consistent with our theoretical models and practical experience with this novel implementation strategy. In addition, LRC was developed in order to maximize its scalability by utilizing virtual observation, but we do not yet have the data to comment on the cost of LRC.
Conclusions
LRC, a form of intervention-specific practice facilitation and coaching, is a novel and scalable implementation strategy for CoCM, with potential to enhance the uptake and sustainment of this complex intervention. It builds mastery in core CoCM skills and, through video conferencing technology, can be delivered with broad reach and low cost by content experts. Challenges to CoCM implementation and to the uptake of offered implementation support included incomplete teams and nonadherence to the recommended LRC schedule, respectively. Key principles of effective population-based mental health care were consistently addressed in LRC feedback. Further examination of these findings and of associations between LRC and implementation/patient outcomes will help refine LRC and support the dissemination of effective mental health treatments in primary and prenatal care.
Although this intervention is being tested in the context of perinatal CoCM implementations, these findings are potentially applicable to CoCM in other patient populations as well as to implementation of other complex interventions.
Acknowledgments
This study was presented in part at the Mental Health Services Research Conference, August 1–2, 2018, Bethesda, Maryland, and at the annual meeting of the Academy of Consultation Liaison Psychiatry, November 13–17, 2018, Orlando, Florida.
This study was funded by National Institute of Mental Health grant 108548.
The authors thank Mindy Vredevoogd, M.S., and Tess Grover, B.S., for their help with the figure.