Medicaid is the largest U.S. payer for substance use disorder treatment (
1). Its coverage of medications—such as buprenorphine, methadone, and naltrexone—for managing opioid use disorders varies by state (
2,
3). Extended-release (XR) buprenorphine may improve treatment retention and reduce drug diversion (
4). In the year after the XR-buprenorphine implant was approved by the U.S. Food and Drug Administration (FDA), only 1% of substance use disorder treatment facilities offered it (
5). Using the 2018 National Survey of Substance Abuse Treatment Services (N-SSATS), I report on the differences among U.S. states and the District of Columbia in the availability of the XR-buprenorphine implant and injection (FDA approved in 2017) for Medicaid-covered patients over a 1-year period (2017–2018).
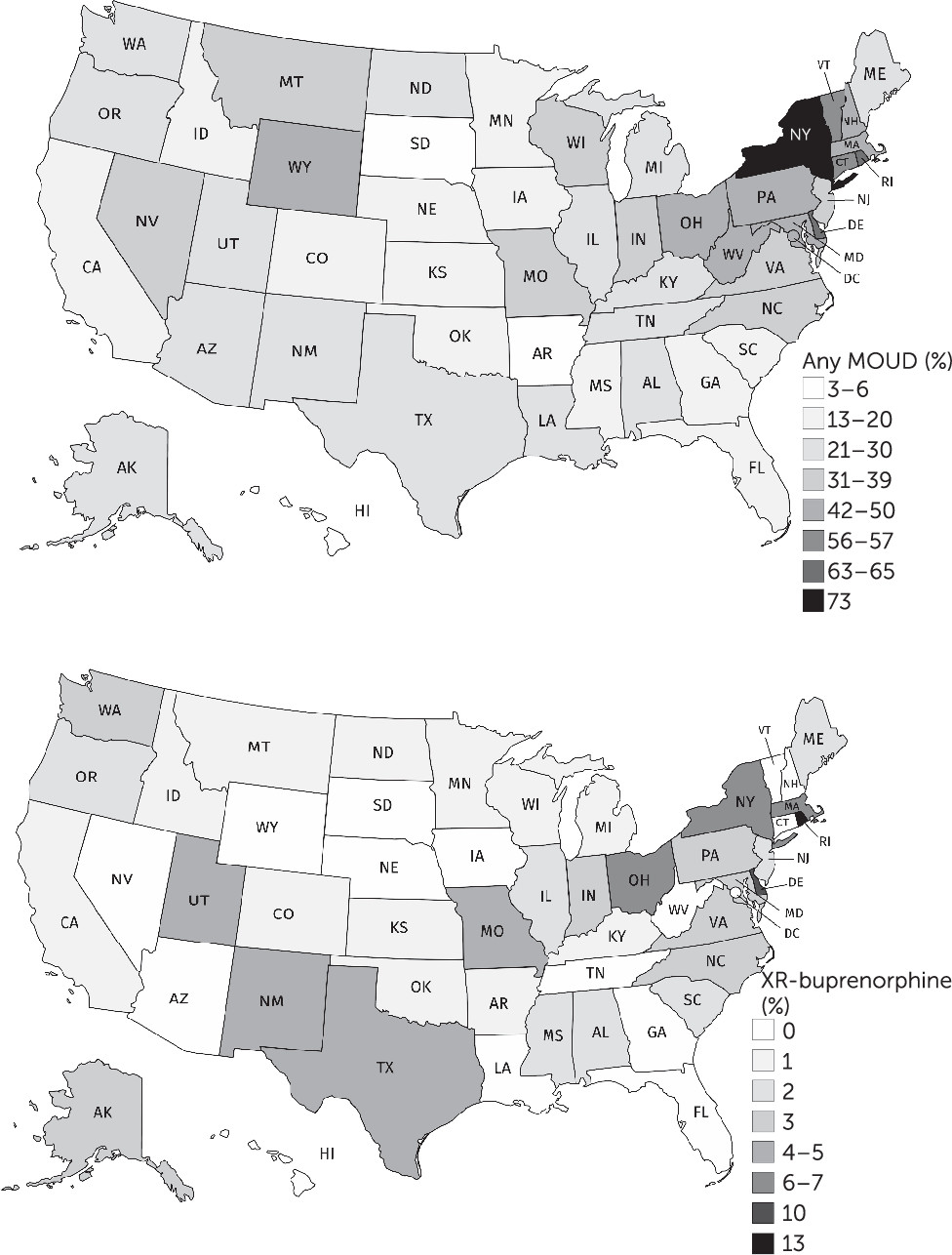
Data from the 2018 N-SSATS and drug-specific coverage by state-administered Medicaid were used to map the availability of general medications for opioid use disorders (MOUDs) and of XR-buprenorphine (implant or injection) at substance use disorder treatment facilities in all 50 U.S. states (
3). Statistically significant differences in the proportion of facilities offering each modality in 2018 versus 2017 were evaluated with chi-square tests at a significance level of α=0.05. Analyses were conducted in Stata 16, and maps were created with MapChart.net.
From 2017 (N=13,481 substance use disorder facilities) to 2018 (N=14,691), the proportion of U.S. substance use disorder treatment facilities offering any MOUD significantly increased, from 37.9% (N=5,110) to 42.5% (N=6,246) (p<0.001). The proportion of facilities offering the XR-buprenorphine implant significantly increased, from 1.1% (N=152) to 2.0% (N=296) (p<0.001), as did the proportion offering extended-release naltrexone (from 23.7% [N=3,192] to 28.4% [N=4,174], p<0.001). Data on XR-buprenorphine injections were first collected in 2018, and 4.0% (N=588) of the treatment facilities offered it. Overall, 65.9% (N=9,675) of facilities accepted Medicaid; of these, 45.5% (N=4,404) offered at least one MOUD, and 4.3% (N=414) offered XR-buprenorphine. The proportion of substance use disorder facilities with Medicaid-covered medications for opioid use disorders varied widely by state, from 3% (N=2) in South Dakota to 73% (N=654) in New York (
Figure 1). Sixteen states had no substance use disorder treatment facilities where a Medicaid-covered client could receive XR-buprenorphine, including 10 states where Medicaid does not cover XR-buprenorphine. In District of Columbia, Hawaii, New Hampshire, South Dakota, Vermont, and West Virginia, Medicaid covered XR-buprenorphine treatment, but none of the facilities that accepted Medicaid offered XR-buprenorphine injection or the implant.
Discussion
Fewer than half of substance use disorder treatment facilities in the United States currently offer any MOUDs, but the substantial increase in availability of these medications from 2017 to 2018 is a positive step. The XR-buprenorphine injection had relatively higher early availability than the implant, but its absolute availability remained low, both overall and for Medicaid clients. Among the states where Medicaid covered XR-buprenorphine for an opioid use disorder, some required preauthorization or step therapy, whereas in others the same medication was available without prior authorization. This heterogeneity was not captured in this analysis, but the broad picture of XR-buprenorphine availability in the United States highlights scale-up opportunities for this medication that should be realized by policy makers, facilities, and clinicians.