Schizophrenia is a severe and persistent mental illness that affects approximately 1% of the U.S. population, or roughly 3.7 million people (
3). This diagnosis predicts poor health outcomes, including a mortality rate that is 3.5 times higher than the rate in the general population (
4). It also predicts poor quality-of-life outcomes, including educational attainment and gainful employment (
2). Consequently, schizophrenia imposes a significant societal economic burden. In 2013, the estimated economic cost of managing schizophrenia in the United States totaled $155.7 billion (
5). Therefore, there is burgeoning interest in interventions to optimize the management of FEP and schizophrenia.
Results
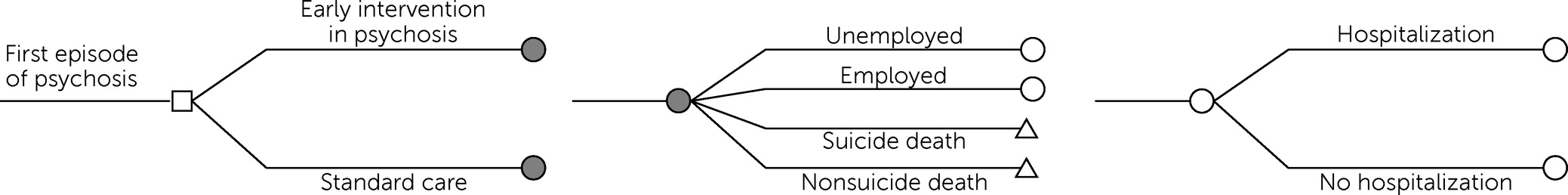
In the base case analysis, patients with FEP who underwent early intervention were estimated to have 12.2 hospitalizations over the course of their remaining life expectancy, whereas patients who underwent standard care were estimated to have 15.4 hospitalizations (
Table 2). Employment estimates were 19.1 years for patients in early intervention and 16.4 years for patients in standard care. From a health care sector perspective, early intervention was estimated to have higher total lifetime costs and QALYs, with an ICER of $51,600 per QALY, compared with standard care. From a societal perspective, early intervention had greater total lifetime discounted QALYs and lower costs than standard care and was therefore predicted to be a cost-saving strategy.
When the risk ratio for hospitalization was updated to an estimate that accounted for publication bias (i.e., less favorable for the early intervention strategy), the results for each base case scenario were reevaluated. From the societal perspective, early intervention had greater total lifetime discounted QALYs and lower costs than standard care; therefore, it was predicted to be a cost-saving strategy but less so than the original base case scenario. From a health care sector perspective, early intervention had higher total lifetime costs and QALYs, with an ICER of $60,980 per QALY, compared with standard care (see the
online supplement).
Table 2 also displays the cost-effectiveness results for a variety of scenario analyses. In a scenario analysis from the societal perspective, where absenteeism and presenteeism were doubled to evaluate an expected worse productivity for patients with psychosis (because our base case estimates were derived from individuals with depression), early intervention was predicted to save costs compared with standard care. When suicide rates were adjusted equally for both interventions (i.e., the suicide rate of standard care was applied to both strategies), early intervention remained a cost-saving treatment but less so than the adjusted productivity scenario. In an equal–suicide rate scenario for our health care perspective, early intervention had an ICER of approximately $197,000 per QALY.
Use of a 10-year analytic time horizon did not change our main cost-effectiveness results for the societal perspective (early intervention remained a health-improving and cost-saving strategy) or the health care perspective (the ICER for early intervention remained between $50,000 and $100,000 per QALY). With a 5-year time horizon, the ICER for early intervention changed to $37,270 per QALY for the societal perspective and $136,920 per QALY for the health care perspective (see
online supplement). In one-way sensitivity analyses, the cost-effectiveness of early intervention compared with standard care was sensitive to the effect of early intervention on suicide, the costs of standard care and early intervention clinics, the relative risk of early intervention on employment, and the utility (health-related quality of life) of unemployment (see
online supplement).
Figure 2 shows the cost-effectiveness acceptability curve results for the probabilistic sensitivity analysis. The early intervention strategy was likely to be optimal in 90.6% of probabilistic sensitivity analysis iterations when a cost-effectiveness threshold of $100,000 per QALY was used. A figure showing the cost-effectiveness acceptability curve results for the probabilistic sensitivity analysis for the base case scenario, which includes an adjusted risk ratio for hospitalization to account for publication bias, is available as an
online supplement to this article. The early intervention strategy was likely to be optimal in 87.8% of probabilistic sensitivity analysis iterations when a cost-effectiveness threshold of $100,000 per QALY was used.
Discussion
We developed a decision-analytic model to compare two clinical treatment strategies for the management of FEP among young adults. From a societal perspective, early intervention saved costs (i.e., yielded more QALYs at a lower cost). Additionally, in scenarios where productivity was adjusted with increased absenteeism and presenteeism, and where we removed the effect of early intervention on suicide, the early intervention strategy remained a cost-saving strategy relative to standard care. From a health care perspective and a willingness-to-pay threshold of $100,000 per QALY, early intervention was cost-effective with an ICER of approximately $51,600 per QALY. When the beneficial effect of early intervention on suicide was removed, early intervention was no longer cost-effective, with an ICER of $197,000 per QALY.
Our results were also sensitive to using a relatively short analytic time horizon (5 years rather than 10 years or lifetime) in combination with a health care perspective (ICER of $136,920 per QALY for early intervention vs. standard care); however, early intervention had an ICER of <$50,000 per QALY when an analytic time horizon of 5 years in combination with a societal perspective was used.
To our knowledge, this model-based economic evaluation of early intervention in psychosis is the first to use U.S. data including costs, where available. It is also the first to consider a lifetime horizon from a societal perspective, include data from the first retrospective cohort study evaluating the effect of early intervention in psychosis on suicide outcomes (
13), and conduct a scenario analysis including estimated adjusted productivity for people living with a primary psychotic illness. The only other model-based, comparative cost-effectiveness analyses for early intervention in psychosis were performed in the United Kingdom (
22,
23). These studies found early intervention to be cost-effective but had data limitations and methodological challenges. In Perez et al.’s (
22) study, the cost of “treatment as usual” (or “standard care”) was assumed to be zero, which is unlikely; we focused on comparing the true incremental costs of early intervention with standard care in our cost-effectiveness analyses. Park et al. (
23) built three decision-analytic models to evaluate early intervention versus standard care for employment and education, as well as homicide and suicide. The authors also used European trial-based and observational data, and their time horizon was limited to the effectiveness data available (i.e., 2 years for employment and education and 10 years for homicide and suicide).
A recent systematic review of cost-effectiveness analyses of early intervention in psychosis identified 16 studies worldwide and revealed that most economic evaluations used a narrow economic perspective (i.e., the health care sector), which fails to account for the broader economic effects of psychosis (
8). In this study, we attempted to address this limitation and included productivity benefits as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine (
15). The inclusion of productivity benefits can have a significant impact on cost-effectiveness results. In one systematic review that evaluated the effects of including productivity costs in economic evaluations of the treatment of depression, approximately 69% of studies ignored productivity costs (
24). For the studies that included them, productivity costs accounted for 60% of total costs per treatment arm. Moreover, the review found that the inclusion of productivity costs (for the studies that did not do so) in most cases substantially affected the incremental costs such that the optimal decision changed. Therefore, the choice of perspective on cost has significant implications on cost-effectiveness results, which, in turn, can affect subsequent decision making. Our findings highlight the importance of including productivity costs or benefits in the economic evaluation of psychiatric disorders, especially because these diseases have broad and ubiquitous societal impact.
To account for the reduction in productivity associated with mental illness, we used estimates for absenteeism and presenteeism from a U.S. study that relied on administrative data of medical treatment, employee absence and disability, and self-report surveys (
16); we found that early intervention was well within conventional standards for cost-effectiveness in the United States. However, the estimates for absenteeism and presenteeism were for “depression-sadness-mental illness,” a vague definition that likely includes minimal to no experiences of psychosis. Because the hallmark symptoms of psychotic illness include difficulty with reality testing and disorganized thought processes (
2), and because the adverse effects of antipsychotic medications include sedation (
25), we would expect lower productivity for persons living with psychosis than suggested by the estimates in the study by Goetzel and colleagues (
16). In our scenario analysis, we therefore doubled the values for absenteeism and presenteeism and found that early intervention remained a cost-saving treatment, although less so than our base case analysis.
One of the most important clinical outcomes for early intervention in psychosis is prevention of suicide. In theory, early intervention should reduce suicide attempts and completions through rapid psychiatric assessment, close follow-up, and better treatment adherence (
6). To our knowledge, there are no trial-based evaluations of suicide outcomes after early intervention versus standard care in the United States. We found the best estimates of these outcomes in a retrospective cohort trial in Hong Kong (
13). The inclusion of these data in this study represented both a strength and a limitation of our model. Without the inclusion of suicide outcomes, our model would have missed important clinical events and misrepresented the lethal risk of psychosis. Conversely, including the Hong Kong data may have not accurately represented suicide outcomes of FEP in the United States and may have introduced bias into our results. To address this limitation, we included a scenario analysis without differential suicide rates between treatment strategies. This scenario was the only one in which, when assuming a limited health care perspective, early intervention was not cost-effective.
Our study had several other limitations. For example, because of the drawbacks associated with the available key data sources that informed the model inputs on the effectiveness of early intervention, our findings may have reduced generalizability. We may have underestimated hospitalization costs because we relied on Medicaid data (because these data were available) instead of Medicare or private insurance data. Our transition probability for employment came from an estimate of employment or school enrollment in early intervention versus treatment as usual (
6); therefore, this calculation may have overestimated employment in this population. As with all model-based, cost-effectiveness analyses, our study required combining data from various sources. Our literature review revealed a paucity of important data for early intervention in psychosis. However, a recent meta-analysis by Correll et al. (
6) provided well-powered estimates for employment and hospitalization rates for patients in early intervention and standard care, which were included in this analysis. The authors included studies from North America, Europe, and Asia. Although the Correll et al. study provides the best available evidence for early intervention leading to a variety of improved outcomes, it may not accurately represent these outcomes in the United States, which was the focus for our study. In practice, when input assumptions are needed for a decision-modeling analysis but are unavailable in a specific country context, the necessary values may be borrowed from neighboring countries or settings with similar characteristics (
15). Typically, these parameters are then varied in a sensitivity analysis to identify threshold values based on relevant cost-effectiveness criteria that would result in no differences among strategies; decision makers can then interpret how plausible these threshold values are for their setting.
Important data gaps remain regarding incarceration, homelessness, caregiver time and productivity costs, social assistance rates, and annual wage earnings for people living with psychosis. Access to these outcomes for early intervention and standard care clinical strategies would provide an opportunity for more comprehensive cost-effectiveness analyses. Until then, decision-analytic models such as ours can synthesize the existing information to inform policy decisions regarding the value of early intervention for psychosis.
Another limitation was our assumption regarding long-term suicide, hospitalization, and employment rates across the life span for patients in this model, namely, that these rates remain constant year to year. We made this assumption for two reasons. First, annualized rates for these outcomes over the life course do not exist. Second, we assumed that early intervention services are not time limited for young adults who later exhibit schizophrenia and other primary psychotic disorders. We assumed that individuals receive psychosocial support in the form of case management across their life span and modeled lifelong treatment costs accordingly.
We chose to build our model with these assumptions because studies have shown that the long-term benefits of early intervention in psychosis all but disappear at 10-year follow-up, likely because of the lack of long-term, follow-up care (
26). These clinical findings combined with this model-based, cost-effectiveness evaluation speak to the importance and economic necessity of long-term support for individuals living with psychotic disorders.