Antipsychotic medication is the cornerstone of symptomatic treatment of schizophrenia and related psychotic disorders, with psychosocial approaches as necessary adjuncts. Traditional drug therapy may have limitations in efficacy and bothersome side effects, in both short-term and long-term use (
1). Clozapine is an atypical neuroleptic drug that has been shown to improve positive as well as negative symptoms when other neuroleptics have failed to do so, and it is not associated with extrapyramidal side effects or tardive dyskinesia (
2).
Studies reporting on the effectiveness of clozapine in long-term use—ranging from one year to 40 years—have focused on clinicians' assessments of changes in symptomatology, side effects, and functioning. Few reports have been published on patients' own subjective experiences with clozapine treatment (
3). Although mental health researchers often view such information as "soft data" of little utility, subjective experiences with medication have been shown to be a powerful predictor of compliance (
4). Moreover, the unpleasant subjective states that patients associate with medication—neuroleptic dysphoria—may be related to comorbid alcohol and drug abuse (
5).
At Hamilton Psychiatric Hospital and its community clinics, our work as specialized care providers for patients with chronic schizophrenic and related disorders serves a catchment population of approximately 1.6 million people in south-central Ontario, Canada. The number of our patients treated with clozapine has been steadily increasing since public funding was begun for this medication in Ontario in 1992.
Although most of these patients showed sufficient objective and functional improvement to justify continued treatment with clozapine, we had no systematic compilation of the patients' subjective experiences with the treatment. Our study objective was to comprehensively survey our patients' own impressions of their clozapine treatment.
Methods
We developed a 37-item questionnaire to elicit the subjective responses of patients in a wide variety of somatopsychic domains. The items were drawn from patients' own reports in our clinical encounters with them, from the Manual for the Assessment and Documentation of Psychopathology (
6), and from the indications and side effects listed in the product monograph for the drug. The questionnaire was designed so that it could be administered in a clinical setting easily and quickly, in an average of ten minutes, and the data readily gathered and entered into a database.
Patients were asked in face-to-face interviews to identify, for each item, the overall change they noticed since they started taking clozapine. Trained interviewers administered the questionnaire verbally in the patients' natural treatment setting. Each response was rated on a 3-point scale (better, worse, and no difference or unchanged). The frequencies of responses were calculated and tabulated for the 37 items.
The sampling frame for the survey was July to December 1997. Our respondents were recruited from the patient population served by Hamilton Psychiatric Hospital in Hamilton, Ontario. The eligibility criteria were a diagnosis of chronic schizophrenic or schizoaffective disorder according to DSM-IV criteria and six months or more on stable doses of clozapine. Of 168 possible patients in this target population, ten refused to participate, and 28 were not available during the study period. Of the 130 patients recruited, 100 were outpatients being seen in two community clinics, and 30 were inpatients of the hospital. All subjects provided written informed consent for the survey.
Results
The survey sample consisted of 99 men and 31 women, with a mean±SD age of 38.7±8.7 years (range, 19 years to 67 years). The daily dose of clozapine was 464±158 mg (range, 125 mg to 1,200 mg), and the duration of clozapine treatment was 34.3±22 months (range, seven months to 98 months).
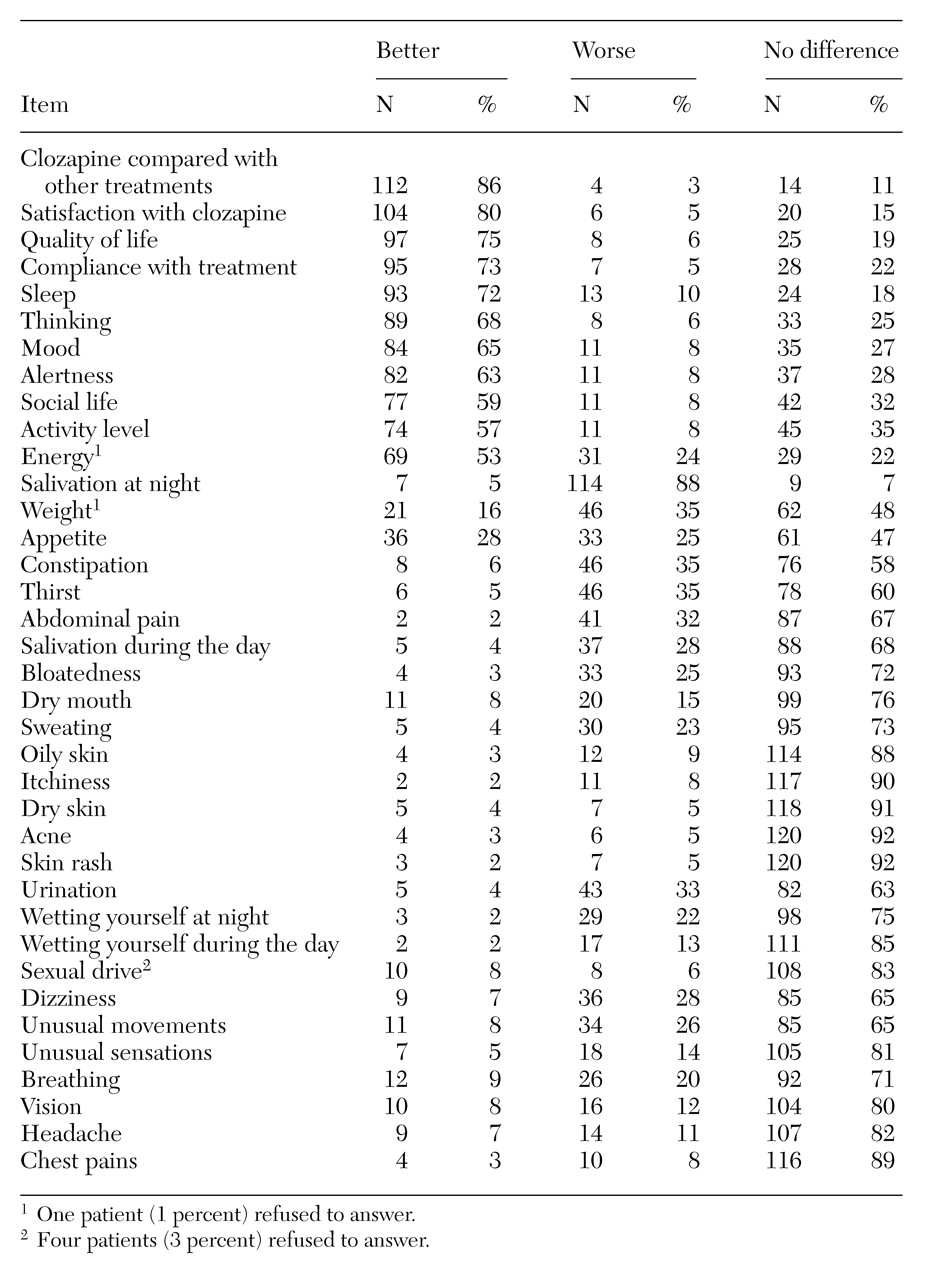
As shown in
Table 1, most of the 130 respondents reported satisfaction with clozapine treatment (especially when compared with past treatments), better quality of life, and an intention to continue using the medication. For the significant target symptoms of treatment with clozapine—thinking, mood, and social and activity levels—the majority of patients reported improvement with the drug.
Among side effects of clozapine treatment, most patients reported a worsening of nocturnal hypersalivation. Substantial numbers reported weight gain and increased constipation, thirst, abdominal pain and bloatedness, urinary difficulties and enuresis, sweating, and unusual movements. However, in most of these categories the majority of patients reported no change.
Discussion
Improvement in the target symptoms of clozapine therapy is an important consideration in the pharmacological treatment of persons with chronic schizophrenic and schizoaffective disorders. It is notable that clozapine's known sedative effect was not reflected substantially in the survey results related to alertness, energy, and activity level. Thus the rewards—items reported as better—of continued clozapine treatment appear to outweigh the many risks—items reported as worse. These benefits may be attributable directly to the effects of clozapine or indirectly to the psychosocial treatments that clozapine may augment (
7).
The strength of our study lies in our direct, wide-ranging questioning of patients to survey their subjective experience of their clozapine treatment. The resulting data have clinical implications and heuristic value. However, this study contains a number of methodologic limitations and sources of bias. Our questionnaire was not tested for reliability and validity. The interviewers were not blind to the treatment condition. There was no control or comparative survey of treatment-responsive patients undergoing long-term treatment with neuroleptics other than clozapine.
A substantial proportion of our respondents, 59 percent, were also taking medications other than clozapine, such as valproate or antidepressants. The reported responses may have been affected by these other medications or by interactions between them and clozapine. Our survey also did not differentiate the study population in terms of adjunctive psychosocial treatments.
Although the reliability and validity of self-report measures in psychiatry may be challenged, patients with schizophrenia have been shown to be similar to physically ill patients in their reporting of quality-of-life items. Such findings point to a certain degree of validity of self-report measures in a schizophrenic population, especially in comparison with affectively disturbed patients (
8).
Conclusions
The results of this survey attest to a subjective efficacy of clozapine. Patients directly reported benefit, satisfaction, and intention to continue taking clozapine, despite the side effect of nocturnal hypersalivation for most patients and other adverse bodily side effects for many. Despite the limitations of our study and the biases that self-report measures may contain, patient-derived data of this sort are clinically meaningful (
9). Systematic exploration of subjective responses to ongoing pharmacological treatment can be usefully included in the array of routinely used outcome measures.