About 5 percent of Americans over 65 years of age live in nursing homes, and the lifetime risk of admission to a nursing home in the United States is 25 to 50 percent (
1). By 2040, as many as four million Americans may be living in nursing homes (
2). Estimates of the prevalence of dementia and other psychiatric disorders in this growing population have ranged from 51 percent to 94 percent (
3,
4,
5,
6,
7,
8,
9). Given the prevalence of neuropsychiatric disorders and the degree of psychopathology in the nursing home population, it is not surprising that psychiatric medications are commonly prescribed in this setting. For example, Rovner and associates (
8) reported that 34 percent of nursing home residents with dementia who were not psychotic were receiving antipsychotic medications. These and other data raised concerns about the appropriateness of the use of psychotropic medications in nursing homes, and the issue came under local and federal scrutiny.
Psychiatric medications have played a controversial role in nursing homes since the expansion of the long-term-care industry in the 1960s. Optimism about the potential benefits of such drugs gave way to overuse, government regulation, and a subsequent change in practices for prescribing psychotropic agents. In the past decade nursing homes and long-term-care settings have been recognized as understudied and complex health care delivery systems. Accordingly, the use and the misuse of psychotropic medications have emerged as potentially important research areas. In this article we review regulatory issues pertinent to the use of psychiatric medications in nursing homes, highlight the major controlled trials of these drugs in this setting, review adverse events associated with psychotropic medications, and propose steps that need to be taken to optimize the use of these medications.
Regulation of psychotropic drugs in nursing homes
The beginning point for regulation of psychotropic drugs in U.S. nursing homes most likely was the introduction of chlorpromazine in the 1950s. This drug, and others that followed, enabled many persons with mental illness to be cared for with considerably less medical and nursing oversight. However, early testing of these drugs did not reveal all of their management complexities and associated side effects (
10,
11,
12,
13,
14).
Around the same time, facilities faced questions about the numbers, qualifications, and adequacy of medical staff and nursing staff. In 1960, a Senate committee noted, "Management of patients in nursing homes by physicians is either lacking or inadequate" (
15).
As the use of psychotropic medications in nursing homes increased, families of residents began to observe rapid deterioration of their loved ones soon after their admission to the nursing home. In 1970, the Senate Special Committee on Aging heard testimony about the "barbaric practice of using tranquilizer drugs as chemical straitjackets for patients who are not in need of such sedation." This led to a series of congressional inquiries that continues to this day (
16,
17).
Efforts by Medicare and Medicaid programs to address these concerns originally centered on self-regulation. In an effort to encourage nursing homes to examine their use of these and other medications, the U.S. Public Health Service provided technical assistance in the form of drug use criteria (
18). The hope was that each facility, through its utilization review committee, would direct and oversee the prescribing and utilization practices of its medical and nursing staff. Unfortunately, this self-assessment and governance mechanism was not successful.
In 1974, Medicare and Medicaid issued new nursing home regulations that required pharmacists to conduct monthly medication reviews and report any irregularities to the medical director and the administrator of the facility. These reviews, which were published in pharmacy and medical journals, provided objective data showing that this review process could significantly improve drug therapy practices. Despite such results, concerns about the misuse of psychotropic drugs persisted.
In 1986, the Institute of Medicine of the National Academy of Sciences issued its report
Improving the Quality of Care in Nursing Homes, which concluded, "Understaffed facilities may make excessive use of antipsychotic drugs to substitute for inadequate numbers of nursing staff" (
19).
In response to the Institute of Medicine's report, the Health Care Financing Administration (HCFA) proposed new rules for nursing homes, and Congress enacted the nursing home provisions of the Omnibus Reconciliation Act of 1987 (OBRA). The HCFA regulations contained provisions addressing the unnecessary use of drugs and antipsychotic drugs. These provisions prohibited the use of chemical restraints and mandated an annual psychopharmacological review that incorporated continuous monitoring of adverse effects. If adverse effects were detected in a nursing home resident, the medication was to be discontinued or the dose reduced according to section F329 in the interpretive guidelines of the regulations.
With the help and support of specialists in many areas of geriatric medicine, HCFA developed extensive guidelines for the use of psychotropic medications. The agency also vigorously promoted nonpharmacological strategies for managing behavioral symptoms through direct mailing of
Managing Behavioral Symptoms in Nursing Home Residents: A Manual for Nursing Home Staff, developed in the department of preventive medicine of the Vanderbilt University School of Medicine (
20).
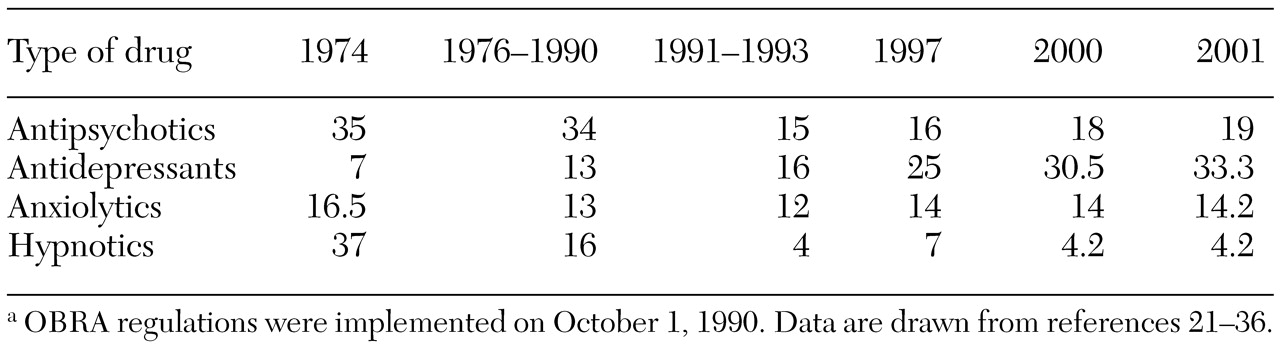
Table 1 summarizes the general trends in psychotropic drug use in nursing homes before and after Congress issued the OBRA regulations, according to various data sources and studies (
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36). The percentages within time periods do not equal 100 because some patients received prescriptions for medications from several drug classes. Comparison across periods is also difficult because of methodological variations among studies, including inconsistent tallying of the use of "as-needed" medications.
These data suggest that after the OBRA regulations were issued, psychotropic drug use fell more in line with the occurrence of actual clinical problems in the nursing home population. Hence the OBRA regulations appear to have encouraged more appropriate use of psychiatric medications in long-term-care facilities. This conclusion is supported by a 1997 survey of randomly selected nursing home administrators in which 77 percent of respondents indicated that inappropriate use of psychopharmacological medications had been reduced in their facility in the preceding two years. Thirty-eight percent of these administrators believed that the OBRA regulations were responsible for these reductions (
37). A number of studies support the inference that these changes in prescribing practices enhanced the functional status of nursing home residents, or at least did not cause a decline in their functional status (
38,
39,
40,
41,
42). These studies support the notion that the risk of adverse consequences of medications is lower for nursing home residents today as a result of the more judicious use of psychopharmacological medications.
Clinical trials of psychotropic drugs in nursing homes
Although the OBRA regulations undoubtedly helped curb the misuse of psychotropic medications and inappropriate prescribing patterns, their focus centered on what should not be done rather than on what should be done. This gap in the knowledge needed to guide best practices can be filled only through carefully conducted clinical trials. In this section we look at the evidence that is available to guide clinical practice. We have selected for review controlled, double-blind trials of psychotropic medications that have been conducted in nursing homes.
Antipsychotics
Two multicenter trials of risperidone have been completed among nursing home patients with significant dementia as indicated by Mini Mental State Examination scores in the range of 6 to 7. The first trial included 625 patients at 40 U.S. sites; 73 percent of the patients had a diagnosis of probable Alzheimer's disease, 15 percent probable vascular dementia, and 12 percent a combination of the two (
43). The mean age was 83 years, and all patients suffered from agitation, psychosis, or both. This randomized parallel-group study compared three dosages of risperidone—.5, 1, and 2 mg a day—and placebo. The authors found that risperidone at a dosage of 1 mg a day was more effective than placebo in reducing some psychotic symptoms and aggression and that it was generally well tolerated. At 2 mg a day, risperidone produced slightly better results but was associated with extrapyramidal symptoms among about 25 percent of the patients. The other multicenter study involved 344 nursing home patients with dementia and either agitation or psychotic symptoms. This randomized parallel-group study compared flexible dosages of risperidone and haloperidol (up to 4 mg a day of each) with placebo (
44). The mean dosage of risperidone was 1.1 mg a day; the mean dosage of haloperidol was 1.2 mg a day. None of the treatments showed a significant effect on psychotic symptoms; however, risperidone was associated with a reduction in some measures of agitation and aggression. The severity of extrapyramidal symptoms did not differ between risperidone and placebo and was less than that associated with use of haloperidol. The recent introduction of a .25 mg dose and a liquid formulation of risperidone offers clinicians some additional dosing flexibility.
Olanzapine has been evaluated in a six-week multicenter, double-blind, placebo-controlled study with 206 elderly U.S. nursing home residents (
45). All patients were suffering from Alzheimer's disease and either psychosis or agitation. Study participants were randomly assigned to receive placebo or olanzapine at a fixed dosage of 5, 10, or 15 mg a day. On average, measures of agitation and psychosis improved with olanzapine at 5 mg and 10 mg a day compared with placebo, but this effect was not evident at 15 mg a day. The major side effects noted were dose-related sedation and gait disturbance. No significant increase in extrapyramidal symptoms was noted at any olanzapine dosage in comparison with placebo. As with risperidone, the recent introduction of a 2.5 mg dose and an orally disintegrating formulation of olanzapine affords clinicians greater flexibility in dosing.
The effects of quetiapine and haloperidol were compared in a ten-week multicenter, randomized, placebo-controlled trial with nursing home residents with dementia and psychosis. Preliminary results have been reported on the subgroup of 284 participants with Alzheimer's dementia (
46). In the active treatment groups, the dosage was slowly increased over a two-week period to a maximum daily dosage of 100 mg of quetiapine or 2 mg of haloperidol, with subsequent adjustments made on the basis of response and tolerability.
The average dosage of quetiapine achieved was 120 mg a day, and the average dosage of haloperidol was 2 mg a day. All three groups experienced an improvement in psychotic symptoms, with no differences between groups. However, both active drugs were superior to placebo in reducing symptoms of agitation. Treatment with quetiapine was better tolerated than treatment with haloperidol and was associated with greater improvements in daily functioning than either haloperidol or placebo.
Antidepressants
Magai and colleagues (
47) studied the effects of sertraline on nursing home residents with late-stage Alzheimer's disease and major or minor depression. According to
DSM-IV criteria, minor depression is characterized by the presence of two to four of nine possible depressive symptoms; one of these must be anhedonia or depressed mood. In addition to the
DSM-IV criteria, the authors used the Cornell Scale for Depression in Dementia (
48), which is the most commonly used instrument for assessing depressive symptoms in this population. Thirty-one participants were randomized in a double-blind fashion to receive placebo or sertraline in a forced dosage titration up to 100 mg a day. In this eight-week trial period, no significant difference was observed between sertraline and placebo; both groups improved over time.
In another study, Streim and colleagues (
49) conducted a randomized, double-blind, ten-week trial of standard and low dosages of nortriptyline—60 to 80 mg a day compared with 10 to 13 mg a day—with 69 nursing home residents who had major depression, minor depression, or dysthymia. The study group included patients with normal cognitive functioning and patients with dementia. Both treatment groups demonstrated significant improvement in symptoms, with no between-group differences in the magnitude of response. However, differences were noted in the dose-response relationship between patients with intact cognitive functioning and patients with cognitive impairment. Among the patients with intact cognitive functioning, there was a significant therapeutic window for clinical improvement as a function of nortriptyline plasma concentrations—between 40 and 111 ng/mL. In contrast, the group with cognitive impairment appeared to demonstrate a leftward shift in the dose-response curve; that is, they responded better to low dosages of nortriptyline than to standard dosages. The authors suggest that depressed patients with more advanced dementia may manifest altered pharmacodynamics that necessitate the use of lower dosages of antidepressants.
Anticonvulsants
Tariot and associates (
50) reported positive results in a comparison of carbamazepine with placebo in a double-blind crossover study involving 25 nursing home patients with dementia and behavioral disturbance. A confirmatory parallel-group study with 51 patients also found positive results using carbamazepine in dosages, on average, of 300 mg a day (
51). This study was terminated early after an interim analysis showed that carbamazepine was associated with reduced agitation and aggression in 77 percent of patients, compared with 21 percent of those taking placebo.
However, concerns about potential interactions of carbamazepine with other drugs have led to the exploration of other anticonvulsants, including valproic acid and its better-tolerated congener, divalproex sodium. Porsteinsson and associates (
52) reported on a randomized, placebo-controlled, six-week study of divalproex sodium involving 56 nursing home patients with dementia and agitation. The average dosage of divalproex sodium achieved was about 840 mg a day. The difference between divalproex sodium and placebo in scores on the agitation subscale of the Brief Psychiatric Rating Scale suggested improvement but was not statistically significant, although it was significant when several covariates were taken into account. Side effects were noted in 68 percent of the divalproex group, compared with 33 percent of the placebo group, but they were generally rated as mild.
A multicenter placebo-controlled nursing home trial of divalproex sodium for treatment of manic symptoms secondary to dementia was suspended because about 25 percent of the medication-treated study subjects experienced sedation. It is likely that the target dosage of 20 mg per kg body weight per day was too high. Among those who completed the study (N=100), no benefit of drug treatment was seen for manic symptoms, but a statistically significant effect was observed on scores of the Cohen-Mansfield Agitation Inventory (
53). A U.S. multicenter, placebo-controlled study of divalproex sodium is under way among nursing home patients with dementia and agitation. There have been anecdotal reports of the use of other, newer anticonvulsants, such as gabapentin, for dementia and neuropsychiatric symptoms, but no prospective studies have been published.
Cholinesterase inhibitors
To date, one study that investigated the use of donepezil in the nursing home setting has been published. Tariot and coworkers (
54) conducted a 24-week, randomized, parallel-group, multicenter, double-blind, placebo-controlled trial of donepezil with 208 nursing home patients who had probable or possible Alzheimer's disease or who had Alzheimer's disease with cerebrovascular disease. The primary aim was to evaluate the effect of this agent on neuropsychiatric symptoms, and the secondary aims were to assess cognitive effects and functioning. Mean neuropsychiatric symptom scores improved over baseline scores for both groups, with no significant differences observed between groups. However, patients treated with donepezil showed a significant advantage in maintenance of cognitive status compared with the placebo group. The overall rates of occurrence and severity of adverse events were similar between the two groups.
Clinical trials summary
Randomized controlled studies in the long-term-care setting indicate that certain anticonvulsants, low dosages of conventional antipsychotics, and atypical antipsychotics can be effective in managing agitation, with effect sizes of about 20 percent for medications that "work." Whether one class is most effective or which medication within a class is best are unknown. Moreover, available studies generally do not address long-term safety and tolerability. To bridge the gap between clinical trials and practice, clinicians sometimes rely on their own clinical experience and on consensus statements and practice guidelines (
55,
56). These guidelines tend to emphasize the use of a class of agents with indications for the predominant target symptoms while minimizing potential toxicity.
Donepezil can help maintain cognitive status as well as cognitive functioning; its effects on neuropsychiatric symptoms require further study. Other cholinesterase inhibitors may provide some benefit to nursing home residents, but none have been assessed in placebo-controlled trials. Similarly, more trials that investigate the treatment of depression among patients with dementia are warranted. As mentioned above, clinicians are guided by limited data when they prescribe antidepressants, and they often base their decisions on their prior experience or on practice guidelines.
Adverse effects of psychiatric drugs
Nursing home residents take more psychoactive medications and are frailer than elderly persons who live in the community. In addition, the incidence of chronic disease in the nursing home population leads to a greater use of other medications. For nursing home residents 65 years of age and older, the most common diagnoses are dementia, heart disease, hypertension, arthritis, and stroke (
57). The average nursing home resident takes six medications daily, and more than 20 percent of nursing home residents take ten or more (
58). Functional disability is also common; more than 80 percent of nursing home residents require staff assistance with three or more activities of daily living (
58).
These risk factors conspire in the development of adverse drug effects. Medical complexity and baseline functional dependence may be a particularly high-risk combination when psychoactive medications are prescribed for the elderly. As a class, psychoactive medications are consistently, but not solely, implicated as a cause of serious adverse drug events among nursing home residents (
59,
60,
61).
Table 2 summarizes data on common adverse drug events associated with psychoactive medication in the nursing home setting, as reported in various studies (
13,
41,
62,
63,
64,
65,
66,
67).
Gurwitz and colleagues (
68) described the incidence and preventability of adverse drug events in a large nursing home cohort. Antipsychotic drugs were associated with the highest incidence of adverse events (23 percent), followed by antibiotics (20 percent), antidepressants (13 percent), and sedative-hypnotics (13 percent). Neuropsychiatric symptoms such as oversedation, confusion, hallucinations, and delirium were the most frequently reported adverse events, accounting for 27 percent of the aggregate; the investigators judged that nearly a third of the neuropsychiatric adverse events could have been prevented. Most preventable adverse events were the result of errors in ordering, such as prescribing the wrong dosage or overlooking an established drug interaction, and careless monitoring, such as failure to respond to laboratory or clinical evidence of drug toxicity.
Gurwitz and colleagues (
69) then sought to identify resident-related factors that were associated with adverse drug events that could be used to identify individuals who were at the greatest risk. Using a prospective case-control study design, the investigators found several independent risk factors for adverse drug events, including being a new resident and receiving anti-infective medications, antipsychotics, or antidepressants.
Given that a considerable number of adverse events in nursing homes are preventable (
68) and predictable on the basis of the above risk factors, it is critical to improve staff recognition, reporting, and monitoring of these events.
Drug interactions may occur when psychoactive medications are prescribed in combination with medications commonly used to treat medical illness. For example, excessive sedation may occur when a benzodiazepine is prescribed concurrently with another sedating drug, such as phenytoin. Displacement of a drug from plasma protein binding sites by another highly protein bound drug, as is the case when aspirin is prescribed for a patient who has been stabilized on valproic acid, can result in a higher free fraction of the displaced drug and possible drug toxicity. The hepatic isoenzyme system is responsible for the oxidative metabolism of many medications; drugs that inhibit or induce various pathways of the cytochrome P-450 enzymes may be implicated in numerous drug interactions. A comprehensive, referenced, and continually updated list of cytochrome P-450 interactions is maintained by David Flockhart, M.D., Ph.D., at Indiana University (http://medicine.iupui.edu/flockhart/).
Current and future considerations
Despite renewed federal and state scrutiny, pharmacy database mining, and a small number of clinical trials, knowledge about the appropriate use of psychotropic medications in nursing homes is in its early years. Given this situation, a number of issues bear deliberation:
• Better psychiatric epidemiological information about the nursing home population and user-friendly tools for assessing behavior and diagnosing psychiatric conditions are needed to ensure the appropriateness of prescriptions for psychotropic medications.
• The regulatory environment must emphasize the need to allocate adequate financial and staffing resources for improving the recognition and management of psychiatric disorders, instead of focusing on punitive fines that do not address the underlying systemic problems.
• Additional randomized placebo-controlled clinical trials are needed that investigate the efficacy of psychotropic medications used alone and in combination to treat well-defined disorders and target symptoms.
• Nonpharmacological behavior management strategies, as well as their use in combination with psychotropic medications, need more study.
• In parallel with efficacy trials, effectiveness trials are needed to assess the influences of a number of factors, including various residential environments, innovative staffing and training strategies, medical and neurological comorbidity, and the neurop medications. Such real-world data could be the cornerstone for the development of useful treatment algorithms.
• Evolving state, federal, and local informed-consent requirements for research involving participants with cognitive impairment may complicate the nursing home research landscape.
• The largest treatment studies have been conducted by the pharmaceutical industry, primarily to obtain efficacy data needed for the FDA approval process. This may affect the degree to which certain public health issues, such as relative effectiveness, can be addressed.
Conclusions
Elderly persons can live in advanced stages of dementia for years, and frequently they reside in nursing homes. Optimal care for these individuals includes addressing all factors related to causes and treatments of behavioral symptoms. Focused research agendas such as those we describe will clarify the consequences of appropriate and inappropriate use of psychoactive medications in this setting. In the meantime, sensible guidelines are available to assist the clinicians who care for these patients (
55,
56,
70).