Schizophrenia is a common and devastating mental illness associated with significant economic and social effects. It is expected that approximately 1 percent to 2 percent of adults in the general population will suffer from schizophrenia at some point during their lifetime (
1). Cost-of-illness studies have estimated that approximately 1.5 percent to 3 percent of national health expenditures in developed countries and 22 percent of the costs of mental illness are related to schizophrenia (
2). The National Institute of Mental Health has estimated that schizophrenia costs the United States about $32.5 billion each year for about 2 million patients with the diagnosis (
3). For comparison, the estimated cost of depression is about $30 billion each year for about 19 million patients with that diagnosis (
3). The majority of direct health costs related to schizophrenia are attributable to hospitalizations for both initial episodes and later relapses. At least half of the relapses can be associated with a lack of compliance with drug therapy, with the remainder linked to issues of treatment efficacy (
4).
The primary purpose of this review is to examine the relationship between noncompliance with drug therapy in the treatment of schizophrenia and the results and economic outcomes of the treatment. This relationship is more difficult to ascertain than may be expected because the economic and clinical effects of new therapies are rarely evaluated simultaneously. Explicit accounting for patients' noncompliance with treatment is also infrequent. This article examines published information on the role and importance of patients' noncompliance with medication therapy in the treatment of schizophrenia. The review is based on a literature search that scrutinized the relationship between noncompliance, relapse rates, and economic consequences.
Methods
We conducted a thorough literature search for studies published between 1995 and 2002 that examined compliance with drug-based therapy in the treatment of schizophrenia. Searches were limited to articles published during or later than 1995 to ensure that the majority of the studies included information on both the typical and atypical drugs used to treat schizophrenia and adequately reflected recent updates in clinical practices.
Searches were performed in MEDLINE (through the National Library of Medicine Gateway), EMBASE, and Current Contents. The primary search terms were "schizophrenia," "compliance," "relapse," and "economic costs." In addition, a variety of Internet-based searches were conducted, and the articles that were identified were reviewed. The initial literature search resulted in the identification of 410 articles, which were then sorted by inclusion and exclusion criteria. To be included in the review the study had to be a clinical study, a meta-analysis, or a literature review; to include a discernible subgroup of patients with schizophrenia; and to examine costs or economic consequences. Pharmacokinetic studies and studies without economic outcomes were excluded. These criteria reduced the number to 25 papers, which were then used as the primary source of evidence for this review.
The results of this review consist of a summary and synthesis of findings. Formal meta-analyses were not performed for several reasons. First, measurements of compliance varied among studies. Different studies used various methods of assessing compliance levels, including identifying compliance or noncompliance in terms of study completion or incompletion, study dropout, and other levels of irregular use of prescribed medication. The reasons for not completing the study or dropping out were not generally explained, and both phenomena can be attributed to many possible causes, including adverse events and lack of efficacy. Even if all incompletions and dropouts were related to compliance levels, the studies may not have measured true rates of compliance, because an individual who had not dropped out could be taking only a proportion of the medication prescribed, which would not be full compliance. According to Cramer and Rosenheck (
5), studies that examine compliance with treatment involving antipsychotic medications have the least quantitative designs, compared with studies that examine compliance with treatment for physical disorders.
Second, studies examining efficacy and those examining effectiveness are generally not comparable. Efficacy refers to the effects that a treatment has in a tightly controlled clinical setting, and effectiveness refers to the effect observed in the "real world," which is complicated by the everyday lives of ordinary people and the quality of care provided in a nonresearch setting. The results or outcomes in these two types of study often differ tremendously. In research on schizophrenia, a large portion of clinical studies are completed in a relatively controlled environment, such as an inpatient setting, and are completed over a relatively short time, usually a one-month to a one-year period. In reality, the treatment of schizophrenia is a long-term process that usually occurs in a wide variety of settings, from institutions to the streets where homeless persons live. Compliance rates have a wide disparity depending on the treatment setting (
5). For example, outpatients are apt to be twice as noncompliant as inpatients (
5).
In addition, the limited study periods in many clinical trials of treatments for schizophrenia may not capture the full effects of the treatment. In general the benefits of such treatments and their clinical outcomes and costs are not fully evident until more than a year after treatment initiation. An extended period of treatment may be necessary before the reduction of side effects, decrease in treatment costs, and improvement in the patient's quality of life become apparent, especially with the use of the newer atypical drugs (
6).
The results of the literature search are summarized in four subsections that address treatment compliance, effect of noncompliance on relapse rates, cost of relapse, and cost effects of noncompliance.
Results
Treatment compliance
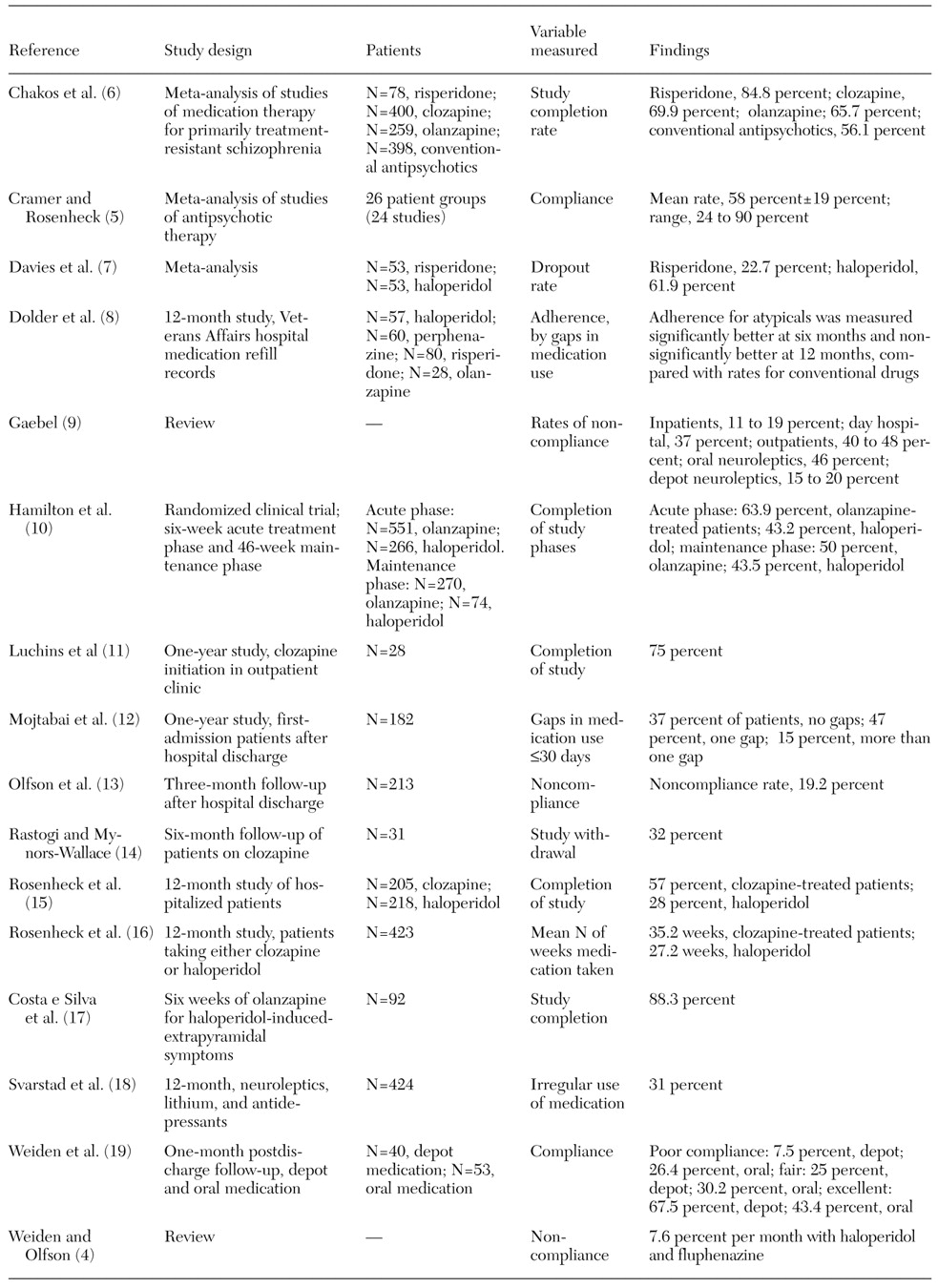
We identified 16 published papers that consider levels of compliance with medication therapy in the treatment of schizophrenia (
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19) (
Table 1). Despite the wide disparity of measurement types and settings in the studies, which preclude quantitative comparison, several trends could be seen in compliance rates. Although the numbers are not directly comparable across studies, study completion rates were better when the newer drugs were utilized. Reported completion rates ranged from 28 percent to 43.5 percent for haloperidol, 50 percent to 65.7 percent for olanzapine, 57 percent to 75 percent for clozapine, and 77.3 percent to 84.8 percent for risperidone (
Table 1).
Although the dropout rate is not an ideal proxy for noncompliance, noncompliance can be considered a significant contributor to dropouts due to lack of efficacy or symptom re-emergence. As
Table 1 shows, studies with longer time horizons have lower completion rates—and hence lower compliance rates. For example, completion rates for studies of olanzapine treatment ranged from 63.9 percent to 88.3 percent at six weeks and 50 percent to 65.7 percent at longer-term follow-up. Similarly, reported completion rates in studies of haloperidol were 43.2 percent at six weeks and 28 percent to 43.5 percent at longer-term follow-up.
In addition, the rate of irregular medication use or the presence of gaps in medication use—another proxy for noncompliance—was reported to range from 31 percent to 62 percent (
Table 1). Noncompliance rates, measured in a variety of ways, were reported to range from 37 percent to 56.6 percent in uncontrolled settings and 11 percent to 32.5 percent in controlled settings (
Table 1).
Effect of noncompliance on relapse rates
Even though many patients respond well to antipsychotic treatment in a first episode of schizophrenia, the risk of subsequent relapses is generally considered to be high (
6). The majority of patients are expected to experience at least one relapse during the five-year period after a previous episode (
19). An individual patient's risk of relapse, which is dependent on treatment, disease, and lifestyle factors, is difficult to predict and quantify. When a patient stops taking medication, either by personal choice or as a result of a poor match between the patient's needs and treatment options, then a subsequent increase in the relapse rate is highly likely.
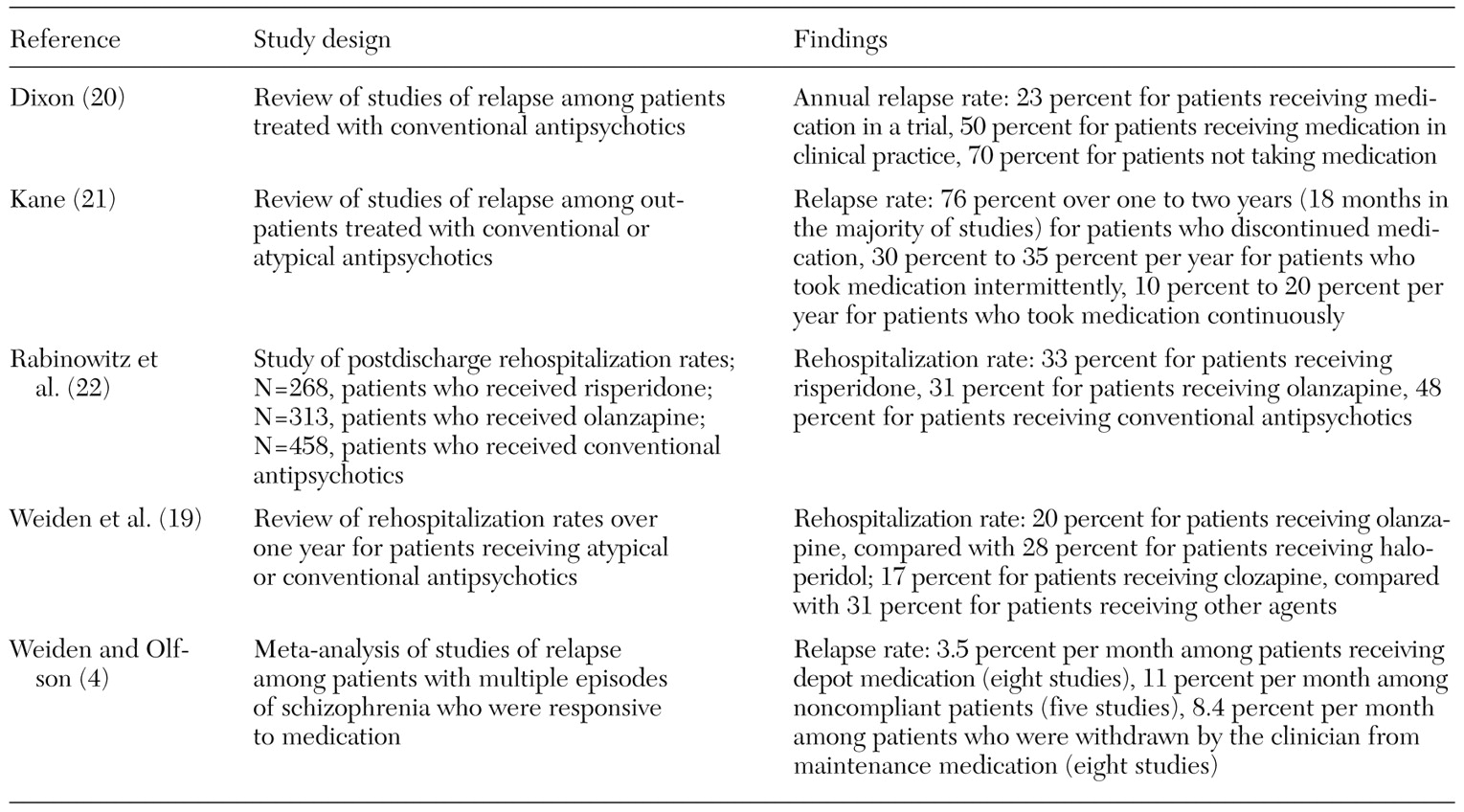
We identified five key papers that considered the effect on relapse rates of noncompliance with antipsychotic treatment (
4,
19,
20,
21,
22) (
Table 2).
Kane (
21) reviewed six independent studies to assess the effect of different treatment interruptions on relapse rates. The review considered the relapse experience of patients who discontinued medication therapy, patients who took maintenance medication intermittently, and patients who remained continuously on maintenance medication. Intermittent therapy has been proposed as a way to retain the clinical benefits of drug treatment while limiting the exposure to side effects through regular breaks from treatment. The review found a mean rate of relapse of 76 percent within the first two years after discontinuation of an antipsychotic treatment, even among patients whose illness was considered to be in remission. Relapse rates for patients who took maintenance medication intermittently were about twice those for patients who took medication continuously (
Table 2).
Weiden and Olfson's review of published data on relapse rates (
4) focused on studies that followed patients whose poor compliance was documented rather than those that reported simple dropout rates. The review suggests an overall monthly relapse rate of approximately 11 percent for noncompliant patients, based on average follow-up periods of six months to two years (
Table 2). This rate is equivalent to a 75 percent annualized risk of relapse for noncompliant patients.
The review by Weiden and Olfson (
4) also considered the results of eight published studies of patients who were treated with optimal doses of depot medication and who were therefore assumed to have a more stable compliance profile. This result was proposed as a proxy for full compliance. The studies' follow-up data, for periods ranging from six months to two years, suggested a comparative annualized relapse rate of about 35 percent (or 3.5 percent per month) for patients with good compliance rates (
Table 2).
This general pattern of relapse data—approximately 75 percent for poor compliance and 35 percent for good compliance—was further confirmed in a separate review by Dixon (
20), who arrived at annual rates of 23 percent and 70 percent as reasonable estimates of benchmark data for patients who were taking and not taking medication, respectively (
Table 2).
Rabinowitz and colleagues (
22) examined relapse rates as the percentage of patients who did not remain in the community. Their reported rates were lower than the rate of general relapses but not as low as those reported by Weiden and associates (
19), who examined relapse rates in terms of the rate of rehospitalization (
Table 2). The rate of relapse requiring rehospitalization, which can be considered a severe form of relapse, was approximately one-third of the general relapse rate.
Cost of relapse
For schizophrenia and for mental disorders generally, the most significant direct costs are related to hospitalization, both for initial episodes of disease and for subsequent relapses. Poor compliance with medication treatment is likely to result in an increased frequency of relapse, more intense symptoms, and longer inpatient stays. These factors that suggest poor compliance leading to relapses of schizophrenic symptoms results in high costs in direct health services.
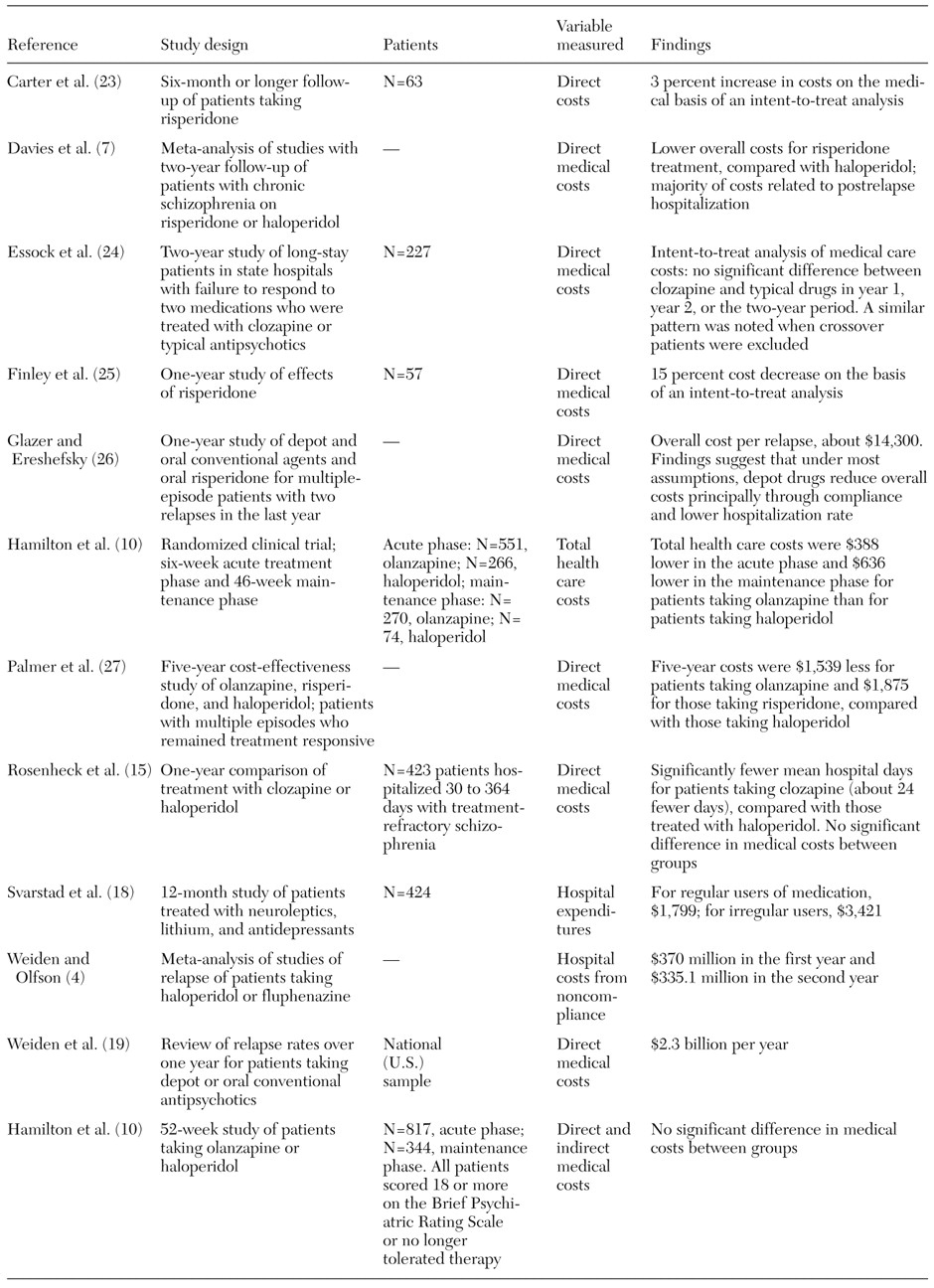
We identified 12 published studies reporting overall hospitalization rates or costs, or both, over a range of time periods for patients with schizophrenia (
4,
7,
10,
15,
18,
19,
23,
24,
25,
26,
27) (
Table 3). Of the 12 studies, only four attempted to place an overall dollar cost on a single relapse (
4,
16,
26,
27). These studies provided resource use and unit cost data that suggest a range of estimates for relapse costs of between $10,000 and $26,000 per episode. Variation in these costs was mainly the result of assumptions about the amount of community-based residential care that is used after discharge from an acute setting and reflected differences between studies in the context of health care. In the findings that were limited to costs for inpatient care, these studies suggested a baseline cost of relapse of about $9,000 to $16,000 per incident.
The detailed study by Glazer and Ereshefsky (
26) provides a further breakdown of the costs of relapse, suggesting that hospitalization represents around 85 percent to 95 percent of the relapse costs, with the remainder distributed among the costs of increased drug dosages, additional clinic follow-ups over the subsequent year, and general case management.
Taken together these studies confirmed that because of high hospitalization costs, relapses place a significant clinical and economic burden on the patient and health care providers.
Estimating the cost effect of noncompliance
Given the high cost associated with episodes of relapse of schizophrenia, the primary economic effect of noncompliance is likely to come from the resulting increases in relapse rates. Although a number of economic studies have compared the cost-effectiveness of antipsychotic medication, very few published studies have explicitly considered the level of additional cost attributed to poor drug compliance alone. We found five studies in our literature review that either estimated the direct cost-benefit from improved levels of drug compliance in schizophrenia or attempted to bring explicit measures of compliance levels into an overall economic consideration of a antipsychotic drug therapy (
4,
18,
10,
26,
27) (
Table 3).
In the most commonly referenced paper related to the cost and economics of noncompliance in schizophrenia, Weiden and Olfson (
4) provided a model using standard survival analysis techniques, based on monthly time periods, to demonstrate the separate effects and cost consequences of treatment withdrawal due to poor efficacy and noncompliance. The analysis was based on data for a cohort of patients followed from discharge who had an estimated 7.6 percent monthly risk of noncompliance. The monthly risk was based on published data related to real-world clinical practice. A best-case scenario was defined as a situation in which patients were expected to comply fully with the medication therapy. The relapse rate for this scenario was based on relapse rates for patients receiving depot medication. This study suggested an estimated 20 percent absolute risk of relapse over two years that can be specifically linked to poor compliance. The annual postdischarge relapse-free rates for the two-year period were 50 percent and 65 percent, respectively, which match the general expectations in the literature (
4).
The study used data on annual U.S. hospitalization costs for schizophrenia (estimated at a total of $2.3 billion) to estimate an average cost per relapse of approximately $9,200 (for an average 22-day length of stay). Combining these figures, the cost of noncompliance with medication therapy was estimated to be about $705 million over the two years. The relative costs of noncompliance compared with costs due to lost efficacy increased in the second year after discharge, reflecting the fact that noncompliance is an ongoing risk to the patient (
4).
A second, more recent study, by Svarstad and associates (
18), used claims data to document hospitalizations for patients with schizophrenia or bipolar disorder. In a cohort of 619 patients with schizophrenia, 31 percent had irregular use of medication, based on 12-month pharmacy records showing whether the patient picked up the prescribed medication. This level of noncompliance was similar for the two diagnostic groups.
Although the estimate of compliance was not very precise, use of hospital services was greater for the patients with irregular medication use than for the patients who picked up their medication. Approximately 33 percent of irregular users were rehospitalized for schizophrenia during the year, compared with 18 percent of those who received their medication. The mean annual costs of hospital expenditures per patient with schizophrenia were about $3,500 and $1,800 for irregular and regular medication users, respectively, a significant difference (
18). Given that the average hospitalization cost for irregular users was $3,500 and only one-third were hospitalized, the cost of hospitalizing a patient is roughly $10,000. This estimate is similar to the estimates suggested by Weiden and Olfson (
4) and others (
26).
A third study, by Glazer and Ereshefsky (
26), used decision analytic modeling techniques to explicitly consider the potential economic effects of different noncompliance risks for alternative antipsychotic medications by linking levels of compliance to relapse rates. Patients whose data entered the model were assumed to have a history of rehospitalization for return or worsening of the symptoms of schizophrenia. These patients are commonly identified as "revolving-door" patients and are those most likely to have relapses. Moreover, these patients have the greatest potential for reductions in use of hospital services.
The effects of depot and oral forms of conventional antipsychotic medications were compared with those of oral risperidone. Annual probabilities of compliance were estimated from the published literature at 80 percent for depot drugs and 50 percent for conventional antipsychotics. Clinical experience suggested a corresponding 65 percent probability of compliance for risperidone, and this estimate was subjected to sensitivity analysis. Hospitalization for relapse was assumed for 10 percent of compliant patients and 55 percent of noncompliant patients, irrespective of the antipsychotic medication used. Direct medical costs were calculated for each drug option. The annual costs of traditional oral, depot, and atypical treatments were estimated at $152, $978, and $2,472. The cost for a relapse was estimated on the basis of the assumption that relapsing patients would experience about 44 days of inpatient care at a cost of about $14,300 per year (
26).
Glazer and Ereshefsky's findings (
26) suggested that a depot form of medication resulted in lower medical costs over the year compared with oral risperidone or conventional oral drugs. Only when compliance rates for risperidone approached those of the depot medication (at 80 percent ) did this situation shift—at which point the drug acquisition cost differential became a critical economic issue. When compliance was equal at 80 percent between a depot formulation and risperidone, a drop in the price of risperidone of 18 percent is necessary for the overall treatment costs to become comparable.
A decision modeling study for United States data by Palmer and colleagues (
27) (adapted for the United Kingdom by Almond [
28]) covered a five-year treatment period and moved patients through health states related to disease symptoms and relapse patterns every three months. In this study, relapse was the major cost element, although it is important to note that the patients were allowed to discontinue therapy, with a subsequent increase in risk of relapse. However, the study considered discontinuation due only to adverse effects or lack of response, which excluded other possible causes of discontinuation. Noncompliance was not explicitly used as a factor in relapse.
The studies by Palmer and Almond concluded that over the five-year period the atypical antipsychotic olanzapine was cost-saving and cost-effective, compared with conventional treatment based on haloperidol. Olanzapine provided significant improvements in the average amount of time that a patient scored less than 18 on the Brief Psychiatric Rating Scale and resulted in slightly lower average treatment costs per patient. A range of sensitivity analyses showed that this clinical benefit remained robust to changes in key parameters and that cost increases associated with these parameters remained small (less than 4 percent ) (
27,
28).
Finally, Davies and colleagues (
7) used a simple decision tree framework to explore the cost effects of risperidone over a two-year period. The model included risk of relapse and also used dropout rates from published trials as a proxy measure for noncompliance. Hospitalization was assumed to be required for all patients at three months after discontinuation of therapy. This study concluded that the atypical antipsychotic risperidone provided more favorable outcomes than haloperidol after a two-year treatment period. In addition, the overall costs of treatment were lower for risperidone. These results were robust to the sensitivity analysis the authors conducted.
Discussion
With the increasing influence of policies for reimbursement of expenditures for drugs, many countries have begun publishing guidelines for minimum criteria to standardize methods for and encourage the production of high-quality economic analyses of medication use. These guidelines are most influential in countries such as Australia, Canada, and the Netherlands. More recently, such guidelines have become important in the United Kingdom (through the National Institute for Clinical Excellence) because economic evidence has become a formal requirement in the approval process for new drugs. New and existing treatments for schizophrenia will be judged against the background of these guidelines and methods for economic analysis (
29).
Economic evaluations that are focused on the short term, either by design or by lack of high-quality clinical evidence, are likely to underestimate economic and clinically important outcomes such as relapse rates and long-term compliance issues. Because of the long-term nature of schizophrenia, with its relapsing course and potential for long-term health care costs, short-term randomized controlled trials are unlikely to provide a fair and reasonable assessment of the full economic benefits of a new intervention. Although many of the benefits of antipsychotic treatments occur during the maintenance phase, when they reduce the risk of future relapse, few data from longer-term clinical studies are available for prospective or retrospective economic analysis. This situation is in part due to the lack of a requirement for a long-term clinical focus in the drug registration process.
The continuous development and application of national and international guidelines in economic evaluation will play a role in ensuring that future evaluations of medications for treatment of schizophrenia are more likely to reach general standards of analytical and methodological quality. On the basis of these guidelines, future studies are more likely to use acceptable comparator treatments, take longer time periods into consideration, include assessments of treatment compliance, include an assessment of the effect on quality of life of the disease and the treatment, have a clear definition of costing methods, and include adequate levels of sensitivity analysis for key factors influencing outcomes. The use of modeling as a technique to evaluate the economic effects of treatments seems likely to become more acceptable in situations in which analysis based on clinical study data alone will not capture the necessary economic impacts.
However, formal guidelines alone may not be able to ensure standards for evidence-based care that are sought by health policy makers. A recent review by Neumann (
30) reiterated the point that the generation of pharmacoeconomic data is not an end in itself. A positive economic assessment for a particular treatment, whether for schizophrenia or cancer, does not guarantee that the treatment will become readily available or that making it available will be perceived as an acceptable use of limited health resources. Many issues make the wider acceptance of economic data problematic: Decision makers may not feel able to adequately interpret data. Financial support of drug companies may taint results through suspected bias. Study results may not be available in time to affect decision-making processes. Generalizing results from a specific patient study group may be difficult.
Conclusions
Economic evaluations involving drug-based therapies in schizophrenia are limited, and direct comparisons between atypical antipsychotics are only just beginning to appear. The short-term nature of many studies fails to reflect the likely long-term treatment adherence patterns and relapse experience that would be of most interest. When studies have explicitly included noncompliance as a direct causal factor for relapse, the results make it clear that treatments offering improved compliance should be expected to have a significant economic benefit. This expectation also holds for therapies that improve efficacy generally. The following points should be considered in designing studies that can be used to consider the economic effects of drug treatments for schizophrenia:
• A study period of at least two years, or ideally five years or more, is necessary, in recognition of the longer-term effects of drug treatment.
• Data for the full spectrum of patients in the study should be reported, including data for all patients initially treated, full follow-up data for those patients, and data for patients who switch treatments or withdraw from therapy.
• To minimize rifts between clinical effectiveness and efficacy, data should be collected in prospectively designed randomized studies that have clinical conditions as close as possible to those in actual clinical practice.
• Relapse rates should be recognized as economically important, and attempts should be made to provide country-specific estimates of the effects of relapse on health care resources.
• Naturalistic studies bring into play the importance of recognizing that patients' compliance with medication therapy is a function of the actual drug profile as well as the overall delivery and support of treatment to the patient. Standard clinical practices and measures should be part of the studies.
• Schizophrenia affects individuals in the prime years of their lives, and the associated indirect costs created by the disorder are substantial. These costs should be measured. Even though they are difficult to quantify, the amounts involved would justify the effort.
• Patients' quality of life should be measured. Although this paper has not focused on this aspect of outcome, economic evaluations need to take into account some measure of benefit to patients in terms of the effects of the disease and the treatment on quality of life.
Compliance with drug therapy remains a major clinical challenge in achieving optimal treatment for patients with schizophrenia. Differences in the precise definition of compliance make direct comparisons of compliance data across studies difficult to perform. What does seem consistent is that compliance within the setting of a clinical trial may be as high as 70 percent to 80 percent. This rate may be significantly lower, perhaps as low as 50 percent, in actual clinical practice. Such differences in compliance rates illustrate the difference between efficacy and effectiveness. The lower rates in actual clinical practice highlight the importance of recognizing the diverse factors that influence noncompliance among patients with schizophrenia.
Improved drug pharmacology is only part of the answer for patients with schizophrenia. Patients with schizophrenia deserve personalized support and encouragement along with practical assistance in optimizing the management of their treatment and understanding their illness. Significant improvements in drug treatments may be possible, however, through improved adverse-effect profiles, alternative routes of administration, and overall enhancement of efficacy. Additional support services such as those provided by assertive community treatment programs, including family therapy, community-based services, and general help with compliance strategies, also have a clear role in improving outcome. The continued improvement in economic evaluation methods can help support this process.
Acknowledgments
This study was funded by Novartis. The authors thank Peter Powchik, M.D., of Novartis for comments on an earlier version.