Barriers to recognition, diagnosis, and treatment
Several factors hinder the appropriate recognition of insomnia and its adequate and appropriate management. They include lack of physician education about insomnia, time-constrained patient visits, beliefs among patients and physicians that sleep complaints are not important, the belief that treatments are not effective or cause more problems, and the lack of research evidence that treating insomnia improves outcomes of comorbid conditions (listed in the box on this page).
Physician training. Most clinicians are not well trained with respect to sleep and sleep disorders. A survey conducted from 1990 to 1991 indicated that 37 percent of medical schools were still failing to include structured sleep medicine sessions in their curricula. On average, less than two hours of total teaching time was allocated to sleep and sleep disorders, and only 11 percent of medical students participated in the clinical evaluation of patients with sleep disorders (
5).
Although research in this area is limited, studies have demonstrated that the lack of training in sleep disorders is reflected in knowledge deficits about sleep medicine among primary care physicians (
6). In a study that assessed the sleep knowledge of 580 primary care physicians, none rated their knowledge of sleep disorders as excellent; 10 percent rated their knowledge as good, 60 percent as fair, and 30 percent as poor (
6).
Office visits. The advent of a managed-care-based clinical environment appears to have contributed to perceptions among clinicians that time spent with patients is too short and that patient load is excessive; these were the findings from a survey of physician assistants, nurse practitioners, and primary care physicians in a large group model health maintenance organization (
7). Despite its prevalence, insomnia is rarely discussed in visits to primary care physicians (
8,
9,
10), and questions about sleep may be swept aside in an effort to save valuable office time. Physicians may avoid exploring problems such as sleep difficulties in order to avoid having to deal with issues that could take up more than the normal allotted time for a patient.
Reporting sleep problems or eliciting a sleep history. Deficits in knowledge about sleep medicine and time constraints both contribute to the reticence among physicians to tackle issues related to sleep. In the World Health Organization's international collaborative study on general health care, attendees in 15 primary care settings in several countries found that physicians detected insomnia in less than 50 percent of patients who had insomnia symptoms (
8). A survey by Papp and colleagues (
6) found that time spent counseling patients on the benefits of sleep was less than that spent discussing diet or exercise.
Neglect of sleep difficulties appears to partially be the result of reluctance on the parts of both patients and physicians to discuss sleep. Ford and Kamerow (
10) found that of those in their cohort who described difficulty sleeping only 9 percent reported the problem to a physician. Shochat and colleagues (
9) found that only 30 percent of patients with sleep difficulties seen in primary care clinics had ever spoken with their physicians about a sleep problem; those who did so reported that they—and not their physicians—were the first to raise the subject of sleep difficulties. Almost 36 percent of patients in the chronic insomnia group reported that the physician's recommendation for management was not effective.
Perception of treatments. There is also a reticence among clinicians to treat insomnia on the basis of their misperceptions about the lack of efficacy of existing agents, restrictions on prescribing hypnotic medications, and the perceived risks associated with use of these agents. Historically, studies that have evaluated medications in randomized controlled trials of insomnia lasting more than six weeks are rare; a meta-analysis of studies using benzodiazepines and zolpidem published in 1997 demonstrated that median duration of trials was seven days (
11). Until recently, little was known, therefore, about efficacy and safety beyond this duration of treatment, despite the fact that many patients may require longer-term treatment. FDA labeling states, "Hypnotics should generally be limited to seven to ten days of use, and reevaluation of the patient is recommended if they are to be taken for more than two to three weeks. [The drug] should not be prescribed in quantities exceeding a one-month supply" (
12). These regulations restrict prescription of hypnotics to short-term treatments and make the chronic prescription of sedative-hypnotics difficult.
FDA labeling was initially directed by the National Institute of Mental Health's (NIMH's) clinical guidelines, which have remained unchanged since they were first published in 1984 (
13). These early findings were based on data that suggested the risk of abuse and dependence associated with benzodiazepines, as well as risks of tolerance, withdrawal, and rebound insomnia phenomena (
14). It is important to note that these guidelines are now considered obsolete by the National Institutes of Health (NIH). In referring to the 1984 guidelines, the NIH Web site now states, "This statement is outdated and is no longer viewed by NIH as guidance for medical practice," which highlights the lack of guidance and direction for the physician treating patients with insomnia (
15). These concerns about the risks of benzodiazepines have likely persisted and have even been transferred to newer agents.
Physicians may not be aware of behavioral techniques for treating insomnia, are generally not trained to perform them, and may not have sufficient time with patients to provide these treatments. Furthermore, because of the paucity of therapists trained in behavioral treatments for sleep disorders, most physicians cannot easily refer patients for needed therapy.
Lack of evidence that treatment improves outcomes. Although insomnia is associated with substantial personal and societal impact, no prospective studies have been done to demonstrate that treating insomnia significantly improves outcomes of its associated comorbid conditions. Walsh and Ustun (
16) estimated total direct annual cost in the United States attributable to insomnia, including prescription and nonprescription medications, outpatient visits to health care professionals, and inpatient or nursing home care, to be approximately $12 billion for health care services and $2 billion for sleep-promoting agents. The impact of treatment of insomnia on this substantial financial burden is unknown, as is the impact of improving insomnia on other negative correlates.
People with insomnia report more days of limited activity, more days in bed due to illness, greater health care costs, and a higher incidence of moderate-to-severe occupational role disability than people without insomnia (
17). Insomnia is also associated with greater health care utilization (
17).
Evidence also suggests that insomnia may lead to the development of depression (
18). Insomnia is also associated with an increased risk of suicide in depression (
19) and resistance to cognitive-behavioral therapy (
20). Finally, the strongest evidence for a causal relationship between insomnia and subsequent illness is in bipolar disorder, where sleep loss has been demonstrated to precipitate or exacerbate episodes of mania (
21).
It is important to note that correlations between insomnia and comorbid illnesses, as well their outcomes, do not necessarily imply causality. The lack of data demonstrating that insomnia has an impact on other illnesses and that treating insomnia improves outcomes in other illnesses is an inherent problem in the field of insomnia research, and more studies need to be conducted in this area. Although some physicians may have observed that treating secondary insomnia benefits patients, there is limited evidence that treating insomnia actually improves outcomes in comorbid illness (
22), and this may be another factor in clinicians' reluctance to recognize or treat insomnia.
What is insomnia?
The nature of insomnia itself probably contributes to the difficulties associated with its treatment. Polysomnographic studies of patients with insomnia generally show abnormalities such as prolonged latency to sleep onset, frequent arousals, and reduced amounts of total sleep. However, objective measures of sleep do not always correlate well with the patient's experience of insomnia (
23), which may be partially due to the fact that the function of sleep itself is still unknown, making it difficult to pinpoint which objective sleep abnormalities contribute to the clinical entity of insomnia. There is a tendency to assume that insomnia is a problem of insufficient amount of sleep. Yet epidemiologic studies consistently demonstrate the lowest mortality rates for people who sleep an average of seven hours per night, with increasing risks for individuals who sleep eight or more hours per night (
24). Furthermore, sleep deprivation does not mimic the effects of insomnia; together, these data suggest that a decreased quantity of sleep alone does not constitute insomnia (
22). From a qualitative standpoint, however, data suggest that people with insomnia show increased levels of arousal, evidenced by markers such as increased, higher-frequency electroencephalographic (EEG) activity during sleep and increased metabolism (
25,
26,
27), but it is not known whether this hyperarousal leads to nonrestorative sleep.
Therefore, it is not surprising that polysomnographic assessments of drugs used to treat insomnia, although perhaps helpful for determining differences between drugs, may not reflect the patient's subjective experience of those drugs, but the ideal would be demonstrating efficacy by both polysomnography and patient report. It is thus important for the clinician to recognize that insomnia, as defined by the
DSM-IV, text revision (
DSM-IV-TR) (
28), is a subjective clinical diagnosis, and therefore a patient's subjective report of sleep difficulties should direct management in most cases. Questions about the range of symptoms experienced and how they have altered over time are important. Because insomnia is a patient-reported symptom, rather than a polysomnographically defined disorder, referral to a sleep laboratory for polysomnographic diagnosis should be reserved for cases in which another primary sleep disorder, such as obstructive sleep apnea or periodic movement disorder, is suspected, because these may require greater expertise in sleep medicine.
The
DSM-IV-TR defines insomnia as "difficulty in initiating or maintaining sleep or … nonrestorative sleep" and as "causing clinically significant distress or impairment in social, occupational, or other important areas of functioning" (see the box on the next page) (
28). As such, all these elements should be considered when diagnosing and treating insomnia. The definition of sleep maintenance remains fairly controversial, but it essentially describes the patient's capacity to remain asleep throughout the night and reflects an assessment of sleep quality. Arousals can be both objectively measured and subjectively reported. Problems with sleep maintenance may consist of longer periods of arousal, which patients are generally aware of, or sleep fragmentation with brief EEG arousals (
29), which patients may not be aware of but that still result in the perception of nonrestorative sleep. Currently used metrics include wake time after sleep onset, wake time during sleep onset, and number of awakenings. Ideally, treatment of insomnia should address components of sleep onset, sleep maintenance, sleep quality, and improvement in next-day functioning.
Difficult-to-treat patients
As noted, long-term prescription of medications for insomnia lacks an evidence base, has been discouraged by existing guidelines, and is a concern among physicians (
13). As shown below, no medications are available that treat sleep maintenance symptoms without the risk of next-day impairment. Chronic insomnia, including sleep maintenance problems, occurs more commonly among the elderly (
31), depressed patients (
32), and medically ill populations (
33,
34), including those with chronic pain syndromes (
34). These patients are often viewed as difficult to treat yet are among the groups that have the greatest need for treatment.
Psychiatric disorders. Insomnia is a very common feature of psychiatric disorders (
33). Prevalence rates of insomnia are greatly increased among persons with psychiatric illnesses. A European epidemiologic sample of 1,536 people found that significant insomnia was present in 71 percent, 69 percent, 61 percent, and 32 percent of those with dementia, depressive disorders, anxiety disorders, and alcoholism, respectively (
35). Furthermore, on the basis of epidemiologic studies, up to 40 percent of adults in the general population with insomnia have a diagnosable psychiatric disorder (
10). Associations between insomnia and psychiatric disorders, particularly depression, are even higher in clinical samples. Approximately three-quarters of patients with insomnia presenting to sleep clinics or primary medical clinics meet diagnostic criteria for psychiatric disorders (
36). Overall, insomnia is more strongly associated with depression than it is with any other medical disorder in the primary care setting (
37). Symptoms of anxiety and depression were also strongly associated among children with insomnia in a pediatric clinic sample (
38).
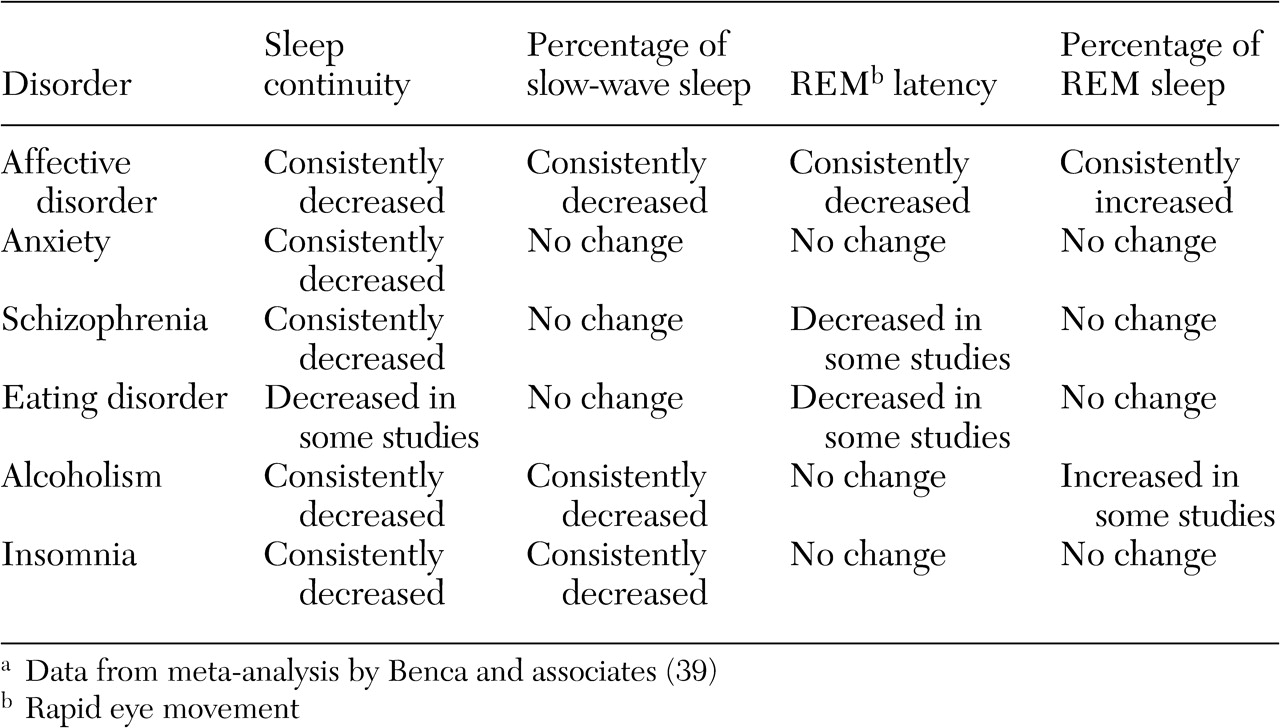
Psychiatric patients present multiple and varied sleep symptoms. Sleep EEG studies demonstrate significant decrements in sleep continuity—prolonged sleep latency, decreased sleep efficiency, and decreased total sleep across the night—in most groups of psychiatric patients compared with control patients without psychiatric disorders (
32,
39) (
Table 1).
The best-studied comorbid psychiatric condition of insomnia is depression. Objective measures demonstrate loss of slow-wave (stage 3 and 4) sleep, frequent nocturnal awakenings, and frequent arousals, all of which may lead to the perception of nonrestorative sleep (
32,
39). Many of these symptoms are consistent with sleep maintenance problems. There is also evidence of disturbed rapid eye movement (REM) sleep architecture in mood disorders, with a reduced latency from sleep onset to REM sleep onset and an increased proportion of REM sleep (
39). Also, insomnia associated with psychiatric conditions frequently lasts for the duration of the illness, and, more important, sleep symptoms have been found to persist despite remission of depression (
40).
Medical disorders. Individuals with insomnia have higher rates of medical illnesses than those without sleep problems. Mellinger and colleagues (
33) determined that 53 percent of adults with serious insomnia had two or more health problems, compared with only 24 percent of those with no sleep difficulties. In addition, Ford and Kamerow's study (
10) found that patients with insomnia used general medical services more frequently, and higher rates of insomnia have been documented among primary care patients than in the general population (
9). There is also evidence that greater severity of sleep disturbance is correlated with worse outcomes among patients who experience pain (
34), that among patients with pain insomnia is correlated with the development of depression (
34), and that the presence of both depression and insomnia contributes to highest pain severity (
41).
Insomnia is also correlated with worse outcomes in a number of medical illnesses, including an increased risk of mortality among institutionalized elderly people (
42), greater disability among stroke patients (
43), and increased risk of mortality among patients with cardiovascular disease (
44).
Elderly patients. Older patients often complain that their sleep is nonrestorative and that they have difficulty staying asleep (
31). Foley and colleagues (
2) found that 30 percent of elderly patients complained of awakening during the night, 19 percent complained of waking up too early, and 19 percent said they had trouble falling asleep. Polysomnographic findings have also suggested that the primary change in the sleep of older adults is an inability to sustain sleep through the night (
45). These findings also suggest that daytime sleepiness among healthy elderly patients without insomnia does not correlate with total sleep time or any sleep stage but is significantly correlated with measures of sleep fragmentation (
46).
Nonpharmacologic management approaches
An American Academy of Sleep Medicine task force reviewed 48 clinical trials and two meta-analyses in an attempt to develop practice guidelines for nondrug alternatives for managing primary chronic insomnia (
47). Its findings indicated that nonpharmacologic therapies produce reliable and long-lasting changes in chronic insomnia sufferers for several sleep parameters. Seventy to 80 percent of patients treated with nonpharmacologic interventions were found to benefit from treatment; however, there was significant variability in the magnitude of treatment response. Stimulus control, progressive muscle relaxation, and paradoxical intention all met the American Psychological Association's criteria for empirically supported psychological treatments for insomnia, and sleep restriction, biofeedback, and multifaceted cognitive-behavioral therapy (CBT) were viewed as "probably efficacious" treatments (
47).
Evidence for favoring pharmacologic therapy over behavioral therapy or vice versa was inconclusive (
48). Comparisons of hypnotic and behavioral therapies suggested that hypnotic drugs produce more rapid improvements in sleep in comparison with behavioral treatments such as relaxation and sleep hygiene education (
48) and that treatment effects over longer periods (four to eight weeks) are similar for pharmacologic and nonpharmacologic therapies, as well as the combination of the two (
49). On the other hand, longer-term outcome studies, such as those of six to 24 months, have indicated that clinical benefit is not as well maintained over time after discontinuation of pharmacologic treatment as it is after cessation of behavioral therapy (
48,
49). Patients who have received combined treatments do not appear to have as good a long-term outcome as those who receive behavioral therapy alone (
50), although reasons for this finding have not been clearly elucidated (
47).
A recent randomized controlled study compared CBT, zolpidem, and the combination of the two therapies among 63 patients with sleep-onset insomnia and impaired daytime functioning (
51). Participants received four weeks of active treatment with 10 mg of zolpidem, followed by 5 mg of zolpidem nightly for one week, and then 5 mg of zolpidem every second night for one week. CBT of 30 minutes per each session was offered weekly for three weeks, with the final session two weeks later. CBT was the most effective intervention in this study in reducing both subjective and objective sleep onset latency after termination of treatment, either alone or combined with pharmacotherapy followed by combination therapy, whereas effects of pharmacotherapy persisted only during acute administration. However, subjectively reported total sleep time showed similar increases after termination of treatment in both the CBT and zolpidem groups; the second largest increase was in the combination therapy group. No between-group differences were found for objective measures. Although these data support the superior efficacy of behavioral treatment over pharmacotherapy, interpretation may be limited by the small samples, the absence of sleep maintenance insomnia or comorbid conditions among these patients, and the failure to assess the effects of treatment on daytime function. More research in larger patient populations is needed to clarify the relative efficacies of the different treatments for insomnia.
Clearly, efficacy coupled with minimal side effects makes behavioral techniques highly recommended for treating insomnia; however, factors such as cost, lack of availability, and potential problems with patient motivation and compliance may make the use of behavioral techniques difficult. Optimal patient- and treatment-related variables have not been clearly defined (
47). A meta-analysis of 59 studies assessing nonpharmacologic treatment of chronic insomnia found that an average of five hours of therapy was provided (
52). The results suggested that stimulus control and sleep restriction techniques were most helpful in producing improvements over an average six-month follow-up period, but sleep hygiene treatment alone was not deemed effective.
Most studies have been conducted with highly trained therapists such as psychologists, who may not be available to a primary care physician's office, and evidence indicates that a lower level of training—a trainee therapist, for example—is associated with worse outcomes (
47). However, several recent studies have suggested that more limited interventions may be helpful to patients with insomnia. CBT was equally helpful when administered in individual, face-to-face treatment, in group therapy sessions, or through brief telephone contact (
53). In another study, two sessions of CBT led to greater improvement for people with chronic primary insomnia than two sessions of generic sleep hygiene education (
54). More research is required to determine the optimal behavioral treatment interventions for insomnia in the primary care setting.
Although sleep hygiene techniques alone do not necessarily lead to consistent improvement in insomnia (
55), they are usually a part of most behavioral treatments for insomnia. Sleep hygiene recommendations are listed in the box on this page, and treatments recommended by the American Academy of Sleep Medicine for the nonpharmacological treatment of insomnia are described in
Table 2.
Pharmacologictreatment approaches
As discussed above, insomnia symptoms tend to change over time. In addition, a survey of a general population sample has shown that a majority of patients with both transient and chronic insomnia have sleep-maintenance problems (
56). In recent years attempts have been made to characterize the ideal hypnotic agent (
57,
58). Attributes proposed for an ideal agent include rapid onset of action and elimination; improved ability to initiate and maintain sleep; improved sleep quality; normal sleep architecture; improved daytime performance and well-being with minimal drug interactions; the absence of hangover effects or unwanted side effects; and no significant potential for tolerance, abuse, dependence, withdrawal, or rebound effects.
Until the advent of the nonbenzodiazepine hypnotics, the most commonly used agents were benzodiazepines. Aside from triazolam, most benzodiazepine hypnotics, which include flurazepam, estazolam, quazepam, and temazepam, have long half-lives, which contribute to their efficacy in maintaining sleep throughout the night but also may result in next-day impairments (
59,
60). The advent of shorter-acting triazolam and the nonbenzodiazepines zolpidem and zaleplon has resulted in more effective sleep-onset agents with minimal next-day residual effects but possibly with decreased efficacy as sleep-maintaining agents (
61,
62,
63,
64).
In addition, no currently approved insomnia agents have been evaluated for treating chronic insomnia, as evidenced by the fact that randomized controlled studies of zolpidem, zaleplon, and approved benzodiazepines have not exceeded four weeks for zaleplon (
62,
64), five weeks for zolpidem (
63), or 12 weeks for temazepam (
65) of continuous use. Although it is currently unknown what duration of continued efficacy would need to be demonstrated in order to support long-term use of an agent for treating insomnia, there is a clear need to demonstrate efficacy of medications over longer periods.
Benzodiazepines. Currently approved benzodiazepines for the treatment of insomnia are flurazepam, triazolam, quazepam, estazolam, and temazepam. Both subjective and objective studies have generally found improvements in sleep maintenance measures, specifically wake time after sleep onset and number of awakenings, with the longer-acting agents like flurazepam, quazepam, and estazolam (
66,
67,
68,
69,
70). Their use, however, is also associated with next-day sedation and impaired cognitive and psychomotor function (
71). Triazolam's efficacy with regard to measures of sleep maintenance is less evident, probably because of its shorter half-life (
72,
73).
Temazepam is the most commonly used benzodiazepine hypnotic (
74). Objective sleep laboratory data regarding the ability of temazepam to improve sleep maintenance as defined by number of awakenings during sleep have been equivocal. Two objective studies did not find significant reductions in number of awakenings with doses of 15 mg to 30 mg of temazepam (
72,
75). Another study (
76) of 48 people with insomnia did show a significant improvement compared with placebo for 15 mg and 30 mg of temazepam on number of awakenings, although significant reductions were seen at only one of the two sites used for the study. Temazepam may also be associated with the development of tolerance (
77). Although adverse events associated with use of temazepam have not been explored to the same extent as those of other benzodiazepines, benzodiazepine use in general has been associated with daytime drowsiness (
71), memory impairment (
71), psychomotor impairment (
71), and risk of tolerance, abuse, and dependence (
72). Temazepam itself has been implicated in causing next-day sedation (
78) and impairment in memory and cognition (
78) and has demonstrated evidence of rebound insomnia on withdrawal and dependence liability (
72). Triazolam has also been implicated in causing rebound insomnia (
79).
Currently available nonbenzodiazepine hypnotics. Evidence for the utility of currently available nonbenzodiazepine hypnotics points to their primary efficacy as sleep-onset, rather than as sleep-maintenance, agents. Once again, longer-term randomized, double-blind, controlled studies that demonstrate efficacy of these agents have not been performed, but safety over the longer term has been demonstrated in open-label studies (
80,
81), with minimal evidence of rebound phenomena. By comparison with benzodiazepines, there has been less evidence of subjective and objective next-day residual effects associated with zolpidem (
4,
63,
82) or subjective next-day impairment with zaleplon, even when the latter has been delivered in the middle of the night (
83).
Fewer clinically important drug-drug interactions appear to occur with zaleplon and zolpidem than with benzodiazepines (
84), which may be related to the differences in CYP metabolism. Whereas triazolam, for example, is biotransformed almost entirely via CYP3A4, the newer agents are biotransformed by CYP3A4 and several other CYP isozymes, which means that CYP3A4 inhibitors and inducers may have less effect on their biotransformation. On the other hand, because these agents are newer, only a few studies have been conducted (
84), and further research is needed.
Zolpidem. Zolpidem is an effective sleep-onset agent and has consistently demonstrated reduced time to sleep onset. It was the most commonly prescribed agent for insomnia in 2001 (
74) despite the absence of studies of nightly use for longer than five weeks (
63). One (
63) of the two (
63,
85) objective studies of zolpidem efficacy in primary insomnia demonstrated significantly improved sleep efficiency; however, neither demonstrated improvement over placebo in reducing wake time after sleep onset or number of awakenings (
63,
85). Another study, conducted with 15 patients with nonorganic insomnia related to neurotic or stress-related disorders, indicated that, although total sleep time improved significantly with 10 mg of zolpidem versus placebo, there were no statistically significant differences in wake time during the sleep period or number of awakenings (
86). These studies (
63,
86) suggest that zolpidem's efficacy in improving sleep efficiency may be related more to its efficacy as a sleep onset agent rather than a sleep maintenance agent. Subjective studies of zolpidem versus placebo have not consistently demonstrated significant improvement in sleep maintenance parameters, such as wake time after sleep onset and number of awakenings (
4,
82).
Although next-day benefits with zolpidem use have not been clearly evaluated or demonstrated (
86,
87), a study by Saletu-Zyhlarz (
86) indicated that there was significant improvement in somatic complaints versus placebo. All other tests of psychomotor function, attention, and memory, as well as subjective reports of well-being, showed no difference compared with placebo. One retrospective case-control study found that use of zolpidem by older people was associated with nearly twice the risk of hip fracture (
88), although evidence generally points to the fact that longer-acting agents are more likely to be associated with falls and hip fractures (
89). The relationship between hypnotic use and falls is complicated by the fact that sleep problems among elderly people are independently associated with an increased risk of falls (
90). Hallucinatory phenomena and other sensory distortions have been reported even with therapeutic doses of zolpidem (
91). Possibly because of its limited efficacy for sleep maintenance problems, patients may take higher than recommended doses or take a second dose during the night, which may increase the risk of both acute side effects and next-day residual effects (
83).
Zaleplon. Zaleplon is a nonbenzodiazepine of the pyrazolopyrimidine class with a very short elimination half-life of about one hour (
62). Several studies have demonstrated that zaleplon is effective in reducing latency-to-sleep-onset insomnia in randomized, controlled, double-blind subjective studies (
62,
64). It is associated with a minimum of next-day side effects or residual sedation, making it a useful agent for sleep-onset problems (
92). However, there are minimal data to support zaleplon's sleep-maintenance efficacy throughout the night when given at sleep onset. In double-blind, placebo-controlled studies, doses of less than 20 mg did not result in significant increases in subjectively assessed total sleep time (
62,
64). Whereas most clinically significant changes, such as reduced sleep latency and increased sleep duration and sleep quality, were seen at the 20 mg dose in studies by Elie and associates (
62) and Fry and colleagues (
64), the recommended dose of 10 mg did not result in increased sleep duration. Only in the Fry study (
64) did 20 mg of zaleplon improve number of awakenings (wake time after sleep onset was not measured), whereas the 10 mg dose had no effect on number of awakenings, suggesting that higher dosing may be necessary for some patients. At present, however, little is known about side effects associated with use of the 20 mg dose.
Trazodone. Despite limited evidence for its efficacy, questions about its side effect profile, and no FDA approval for use as a hypnotic, trazodone is one of the two most commonly prescribed agents for insomnia (the other being zolpidem) (
74). Trazodone's precise mechanisms of action have not been determined; it is believed to be a weak but specific inhibitor of synaptosomal reuptake of serotonin, and its therapeutic effects may also be based on the serotonergic 5-HT
1a, 5-HT
1c, and 5-H
2 receptors (
93). As an antidepressant, trazodone has the advantages of low cost, no restrictions on long-term prescription, and a low abuse potential compared with benzodiazepine receptor agonists (
94). However, the risk of side effects associated with trazodone is not trivial and includes orthostatic hypotension and blurred vision (
95), which increase the risk of falls, especially among elderly patients. Symptoms such as nausea, dry mouth, constipation, drowsiness, headache, and other central nervous system effects also increase morbidity (
95,
96). Priapism is a rare side effect of trazodone, not associated with use of benzodiazepine receptor agonists, and its occurrence is considered a urological emergency (
97).
Given these drawbacks, it is important to ask how effective trazodone is with respect to insomnia in general and sleep maintenance in particular. Surprisingly, considering the drug's popularity, there is a paucity of data supporting its use for insomnia, especially in primary insomnia populations, because trazodone studies have usually been conducted among depressed patients (
98) and have utilized small samples (N≤22) (
98). Objective studies have not exceeded eight weeks (
99). Two (
3,
98) of the three studies (
3,
98,
100) that objectively measured waking time after sleep onset did not show benefit. This finding was also reflected in studies that used subjective measures (
101,
102). Although trazodone showed statistically significant subjective improvements from baseline in ease of falling asleep and quality of sleep, it was also associated with negative effects in terms of ease of awakening and feelings at or after wakening, relative to baseline. This finding suggests that the improvements in sleep onset and quality may be counterbalanced by negative next-day effects. Studies have also demonstrated evidence of declining efficacy after one to two weeks of treatment, suggesting the possible development of tolerance (
3,
4). Trazodone has also been associated with rebound insomnia after withdrawal (
3).
Over-the-counter medications. Sedating antihistamines are frequently used as sleep aids (
103), possibly because of their perceived safety and low cost. A 2002 National Sleep Foundation poll of 1,000 people found that 24 percent of those who reported sleep difficulties used over-the-counter or store-bought sleep aids; 5 percent of those who used over-the-counter drugs reported use every night or a few nights a week (
104). Diphenhydramine, the most widely used over-the-counter antihistamine sleep aid, appears to be superior to placebo in several double-blind, placebo-controlled studies (
105,
106), although neither of these studies used objective polysomnographic measurements. Most studies were of very short duration and involved patients with mild-to-moderate insomnia. No recent controlled studies demonstrate efficacy of diphenhydramine for longer than three weeks for objectively determined measures of sleep maintenance. Diphenhydramine also appears to produce tolerance to its sleep-inducing effects within a few days (
107). Moreover, over-the-counter antihistamines have substantial neurocognitive effects, including next-day sedation (
105) and impaired psychomotor and cognitive function (
108,
109).
The potential for diphenhydramine-related toxicity and drug-drug interactions is substantial (
110). Side effects include urinary retention and blurred vision (
111); orthostatic hypotension, dizziness, and palpitations (
105,
111); increased liver enzymes (
105,
106); and drowsiness, dizziness, grogginess, and tiredness (
105). The available evidence suggests that diphenhydramine and related over-the-counter antihistamines do not represent a viable treatment strategy for long-term sleep maintenance in chronic insomnia.