A national crisis in geriatric mental health care is looming because of the anticipated growth of the population of people over the age of 65 (
1). Research on major depressive disorder (MDD) in geriatric psychiatry has lagged behind that of younger adults. The need to better address issues regarding MDD is highlighted by evidence suggesting that the point prevalence for those over the age of 65, 4.4% in women and 2.7% in men, is considerably higher than estimates from earlier epidemiological studies (
2–
4). Although MDD is highly prevalent, our knowledge about the long-term course of this disorder in elderly patients is limited, and only a few studies have examined this issue prospectively beyond 2 years. Also, less is known about how the course of MDD in elderly patients differs from that of younger adults (
5).
Much of the literature published between the years 1955 and 1994, on the course of MDD among elderly patients age 60 and older, with study sample sizes of at least 20 and a minimum follow-up over 12 months, was reviewed in a metaanalysis (
6). Cole and Bellavance’s review raised serious methodological concerns about many of those studies. None of the studies described their referral patterns. Few studies defined criteria for assignment into categories of recovered or not. Most of the studies did not use systematic assessment or follow-up. The follow-up periods were typically 1 year, which limits the knowledge about the subsequent course. Many of the hospital- or clinic-based studies provided short-term follow-up data of 24 months or less (
7–
14), and six had follow-up over 2 years, but none longer than 4 years (
15–
20). Among those six reports, 27.3% of the depressed elderly patients were well at the end of follow-up; 32.5% had a recurrence after recovery; and 14.2% remained continuously ill. Three of the five community-based studies (
21–
25) were longer than 2 years, reporting 19% of the depressed elderly patients as being well and 27.0% remaining continuously ill (
23–
25).
Since that review, several other investigators using the criteria suggested by Cole and Bellavance have examined this issue in treated samples (
26–
34). Only two clinically-based studies were greater than 12 months in duration. Alexopoulos et al. (
27) examined recovery in individuals over the age of 63 during a 2-year period and found that 29% did not recover. Stek et al. (
33) obtained follow-up data on 155 individuals discharged from a psychiatric hospital with a diagnosis of MDD; of those still living 6 to 8 years after the index episode, only 33% were described as doing well. A number of additional studies have been based on community samples, examining the course of depression in elderly patients. Three studies ranging from 2.6 to 5 years of follow-up with depressed subjects found that the rate of recovery ranged from 46% to 57% (
29–
31). The Longitudinal Aging Study, Amsterdam has provided data on 6 years of follow-up of a cohort of 277 depressed subjects older than 55 years (
34). This study found that 32% had a severe chronic course, and 44% had a fluctuating course, on the basis of the Center for Epidemiological Studies–Depression scale (CES–D) as a measure of severity and the Diagnostic Interview Schedule to confirm diagnosis.
Few of these studies have included a younger cohort as a comparison group. Meats et al. (
11) found that the proportion of those who were well at the end of 12 months was higher in elderly patients. Brodaty et al. (
16) found that, compared with a younger group, recovery rates among depressed elderly patients continued to rise during the 45 months of follow-up. Tuma (
26) and Alexopoulos et al. (
27) also noted a similar outcome between older and younger patients.
Our report relies on the strengths of the Collaborative Depression Study (CDS), which includes specified recruitment criteria, a wide range of subject ages, systematic assessment using standardized and well accepted instruments, up to 15 years of follow-up, and frequent short-interval prospective interviews, to examine the course of MDD in elderly patients. This study overcomes many of the methodological issues raised by earlier reviews. We examined the course of the index episode of MDD and the well period in those who recovered and the treatment received in subjects divided into four groups on the basis of their age at intake: 17–30, 31–50, 51–64, and 65–79 years of age.
Methods
Subjects
Between the years 1978 and 1981, a total of 955 patients who sought psychiatric treatment for a major mood disorder at one of five academic medical centers in the United States (Boston, Chicago, Iowa City, New York City, and St. Louis) were entered into a prospective, observational follow-up study, the NIMH Collaborative Study on the Psychobiology of Depression (CDS). This report examines the first 15 years of follow-up data on the 431 subjects who were diagnosed with MDD at intake and who also had no previous history of mania, hypomania, schizoaffective disorder, chronic intermittent depressive disorder, or minor depression of at least 2 years’ duration at intake. Since the subjects age 65 and over, who are the focus of this report, were all inpatients, we further limited the sample to only subjects who were recruited from the inpatient setting in order to limit sources of bias. This resulted in a subsample that numbered 332 subjects. We obtained written informed consent from the subjects after they received a complete description of the study.
Assessments
The details of the assessment procedures are described elsewhere (
35,
36). All subjects were interviewed at intake by trained research staff using the Schedule for Affective Disorders and Schizophrenia (SADS) (
37). This information was combined with medical records to make diagnoses according to Research Diagnostic Criteria (RDC) (
38). RDC criteria for MDD are essentially identical to those for DSM-IV. We have maintained RDC diagnostic categories in the CDS to maintain consistency of method over the two decades of the project. In the RDC, the primary/secondary distinction is determined solely by the chronology of onset of the illness. Primary MDD in the RDC is defined according to the Feighner criteria, which state that MDD is primary as long as it began before any one of a defined list of non-affective disorders (
39). After intake, subjects were interviewed every 6 months for the first 5 years with the Longitudinal Interval Follow-up Evaluation (LIFE) (
40) and every year thereafter with the Streamlined Longitudinal Interval Continuation Evaluation (SLICE). These follow-up instruments assess the level of psychopathology for each RDC major affective disorder (major depression, mania, schizoaffective mania, schizoaffective depression) on a 6-point scale called the Psychiatric Status Rating (PSR) (
36). A rating of 1 denotes no symptoms of the disorders, and a 6 denotes full diagnostic criteria, with psychosis or severe impairment. In these subjects with MDD, recovery from MDD is defined as beginning with the first of 8 consecutive weeks of no or minimal symptoms (PSR 1 or 2, respectively). Until recovery occurs, a subject remains in an episode of MDD, at a level of psychopathology ranging from PSR 1 to PSR 6. The continuous string of PSR scores for MDD lasting up to 780 weeks (15 years) is the source of data for these analyses on the course of illness.
Treatment
Weekly somatic antidepressant treatment was assessed retrospectively by the LIFE or SLICE. These detailed records of pharmacotherapy were combined by use of a 5-point summary scale called the Composite Unipolar Antidepressant (CAD) scale, which quantifies all antidepressant somatotherapy, including electroconvulsive (ECT) and pharmacotherapy (
40). Equivalent dose ranges were established for each antidepressant. A CAD score of 0 means that no antidepressant somatic treatment was provided for that week. A CAD score of 1 is equal to a daily dose of 1 mg–99 mg imipramine or its equivalent. A CAD score of 2 is equal to a 100 mg–199 mg imipramine-equivalent; 3 equals 200 mg–299 mg imipramine-equivalent; and 4 equals 300 mg-or-more imipramine-equivalent. The study protocol did not influence the treatment provided by the patient’s physician. We specifically looked at the extent of “adequate pharmacotherapy” during the intake episode and first prospectively-observed well period. “Adequate pharmacotherapy” was defined as receiving treatment at a CAD level of ≥2. Psychotherapeutic treatments were not systematically assessed and are not included in these analyses.
Statistical methods
The outcomes of interest in these analyses were time-to-recovery from the intake episode of MDD and subsequent time until the occurrence (prospectively observed) of any major affective disorder (MDD, mania, schizoaffective mania, or depression). The comparisons of interest were the findings in the subsample that was 65–79 years of age at intake (N=32) compared with the three other age-groups (17–30 [N=119], 31–50 [N=118], and 51–64 [N=63]). The number of subjects in each group who, after recovery from their index episode of MDD, experienced the occurrence of a non-MDD affective disorder is small (mania 1, 1, 2, 0; schizoaffective mania: 0, 0, 0, 0; schizoaffective depression: 1, 0, 0, 0, in the 17–30, 31–50, 51–64, and 65–79 age-groups, respectively). Survival analysis was used to analyze time until recovery from the intake episode and, in those who recovered, time to first prospectively-observed recurrence during the follow-up (
41). In the case of the intake episode of MDD, the survival time represents the “time until recovery,” defined as the number of consecutive weeks from intake into the study during which the subject continued to show symptoms of MDD. A survival interval terminated in one of two ways: 1) an episode resolved, or 2) no resolution of episode by the time of final assessment. The latter subjects were classified as censored in the survival analysis. The Kaplan-Meier product limit was used to estimate the cumulative probability of recovery or recurrence (
42). Survival analysis accounts for varying lengths of follow-up and allows us to utilize all the data collected (
43). However, the analyses make no attributions about the cause of loss to follow-up status, but assumes that it was independent of the event of interest (i.e., recovery or recurrence, respectively).
Pearson chi-square and one-way analysis of variance (ANOVA) were used for univariate comparisons of the age-groups on clinical and demographic features. Where the data were ordinal categories (e.g., number episodes of MDD before intake) or failed a Levine test for homogeneity of variances, a Kruskal-Wallis chi-square test was utilized. A two-tailed alpha level of 0.05 was used for each statistical test. The post-hoc procedure described by Conover (
44) was used for statistically significant Kruskal-Wallis tests, whereas Bonferroni-adjusted pairwise comparisons were used for significant chi-square and log-rank tests, and where the Bonferroni denominator was equal to the number of pairwise tests.
Results
Table 1 details the clinical and demographic characteristics of the four groups. The elderly group was significantly more likely than the younger groups to be widowed, separated, or divorced, to have three or more previous episodes compared to the youngest group, to have primary MDD (as defined in RDC as occurring before the onset of any one of a specified list of non-affective disorders, such as phobic disorder or alcoholism), and to have a history of cardiovascular and oncologic illnesses.
During the intake episode of MDD after entry into the study and during the first prospectively-observed well period, the proportion of subjects who received pharmacotherapy at a level ≥CAD 2 (100 mg–199 mg imipramine-equivalents) was quite low for all groups, with no statistically significant difference in the levels of treatment received (Table 2). These low levels of pharmacotherapy occurred in the context of a group of people who, as a whole, had a mean of five previous episodes of MDD.
Course of major depressive disorder
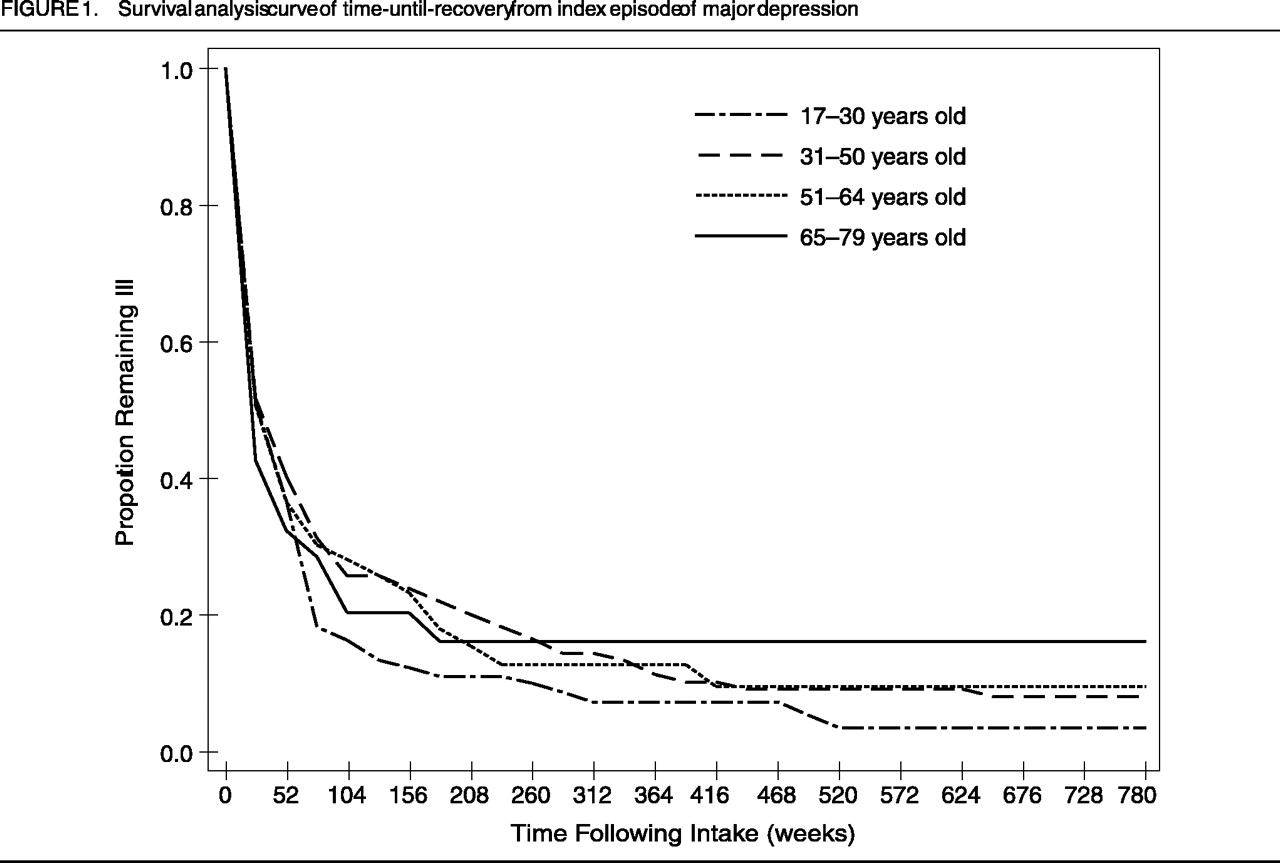
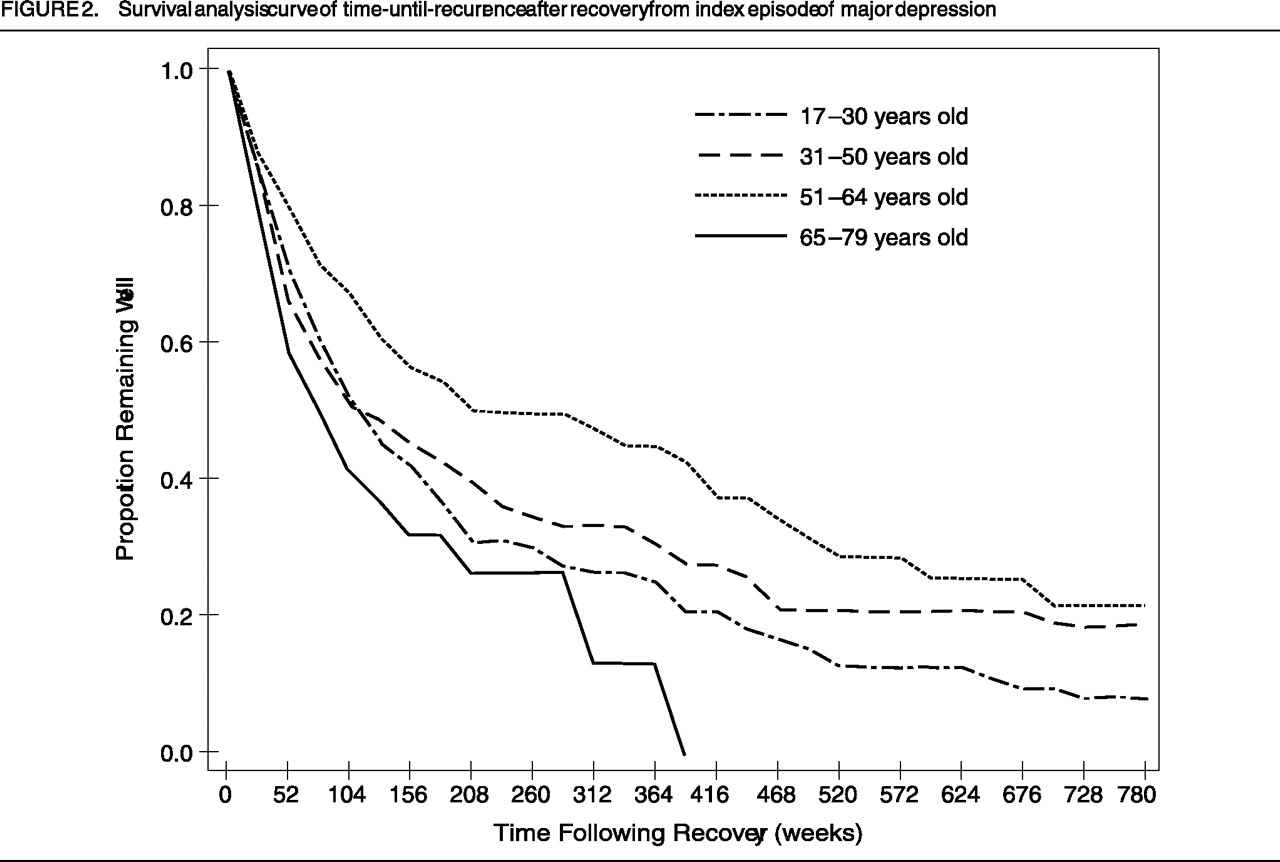
Figure 1 and Figure 2, respectively, present the survival curves for time-to-recovery from intake episode and time-to-recurrence in those who recovered. The median times-to-recovery for the four groups (shown in Figure 2) are similar (log-rank[3]=2.35; p=0.50): 17–30-year-old group: 28 weeks (95% confidence interval [CI]: 16–38 weeks); 31–50-year-old group: 28 weeks (95% CI: 11–45 weeks); 51–64 year old group: 29 weeks (95% CI: 11–47 weeks); and 65–79 year old group: 12 weeks (95% CI: 1–23 weeks). For the subjects who recovered from the intake episode (N=106, 104, 50, and 25, in the respective age-groups), we analyzed the time to the first prospectively observed occurrence of a major affective disorder. The median time-to-recurrence was significantly different among groups (log-rank[3]=9.88; p<0.02): 17–30-year-old group: 118 weeks (95% CI: 69–167 weeks); 31–50-year-old group: 112 weeks (95% CI: 51–173 weeks); 51–64-year-old group: 293 weeks (95% CI: 64–522 weeks); and 65–79-year-old group: 90 weeks (95% CI: 29–151 weeks). Post-hoc analyses revealed that the only statistically significant difference was between the 51–64-year-old group and those 65–79 years old (log-rank[1]=10.24; p<0.008), where the older group experienced a more rapid recurrence. Examination of Figure 1 and Figure 2 provides the proportion of subjects in the respective age-groups who remained continuously ill (Figure 1) or continuously well, once recovered (Figure 2), for the entire follow-up period.
Discussion
This study found that elderly patients, compared with younger groups, had a similar MDD course for time until recovery from the intake episode. However, older subjects experienced a recurrence after recovery more rapidly than the cohort of subjects between the ages of 51 and 64 years. Our study incorporates many of the criteria suggested by Cole and Bellavance (
6) for prospective studies examining the course of MDDin elderly patients. The CDS offers the unique advantage of a long follow-up period relative to previous work. The CDS has specified selection criteria for entry into the study, minimum severity requirements, systematically applied assessment and follow-up, and clear definitions for assignment into categories of recovered or not. Outcome measures were based on specified criteria, and all somatic treatment was recorded.
Despite these advances, the study was limited by the small sample of elderly subjects enrolled at intake; this also precluded a meaningful analysis of predictors of course. Since the study sample has aged since intake, future analyses will be able to incorporate greater numbers of elderly subjects, permitting us to examine clinical and demographic characteristics as predictors of course. Furthermore, since the study was not originally designed to examine the outcome of depression in elderly patients, measures of activities of daily-living functioning and cognitive status were not included. Evaluations of these domains are planned to be included in future assessments. Using clinical samples that are not limited to first lifetime episode has been criticized because elderly individuals with multiple recurrences will have an increased chance of being included in the study; this bias was borne out in this report. In the future, as elderly patients’ sample size grows, we will be able to control for this bias by including it as a variable in our predictor analyses. We looked only at people who presented to academic medical centers for treatment of moderate-to-severe MDD, and they may not represent a general group of geriatric people with MDD.
All subjects in this analysis, including the elderly group, received low levels of somatic antidepressant therapy. It has been shown that adequate treatment of elderly individuals with recurrent MDD markedly decreases the risk for recurrence and that long-term maintenance treatment is superior to placebo in reducing risk of recurrence (
45–
47). These studies and ours suggest that the long-term outcome of MDD in elderly patients, as in younger adults, has a course characterized by recovery and recurrence. Whether or not optimal somatic and psychosocial treatments would ameliorate the course of this disorder in elderly patients cannot be answered by this study. Unfortunately, this study does not provide guidance for the clinician to make decisions about the approach to a specific patient; however, the evidence for a more rapid recurrence after recovery in elderly patients, compared with a younger control group, suggests that most elderly people with MDD, similar to those who are younger, will benefit from continued attention to their mood disorder even after apparent recovery.