The benefit of solid organ transplantation was realized in 1954 when Dr. Joseph E. Murray performed the first successful kidney transplant, with the patient’s identical twin as donor. However, for most patients an identical-twin donor was not an option, and more than a decade passed before immunosuppressive medications were available to conquer the immunological barrier. In 1967, the first successful liver transplant was performed, followed a year later by the first successful heart transplant. Yet despite the fact that the surgical challenges of solid organ transplantation had been overcome, it was not until the early 1980s, with the advent of improved immunosuppression, that organ transplantation changed from an experimental procedure to a standard of care for many types of end-stage organ disease.
In that decade, the National Organ Transplant Act established the framework for a national system of organ transplantation, and the United Network of Organ Sharing (UNOS) was contracted by the U.S. Congress to administer the nation’s only Organ Procurement and Transplantation Network (OPTN) (
United Network of Organ Sharing 2004). Currently, UNOS administers the OPTN under contract with the U.S. Department of Health and Human Services. In addition to facilitating organ matching and placement, UNOS collects data about every transplant performed in the United States and maintains information on every organ type (e.g., wait-list counts, survival rates) in an extensive database available on the OPTN Web site (
http://www.OPTN.org) (
United Network of Organ Sharing 2004).
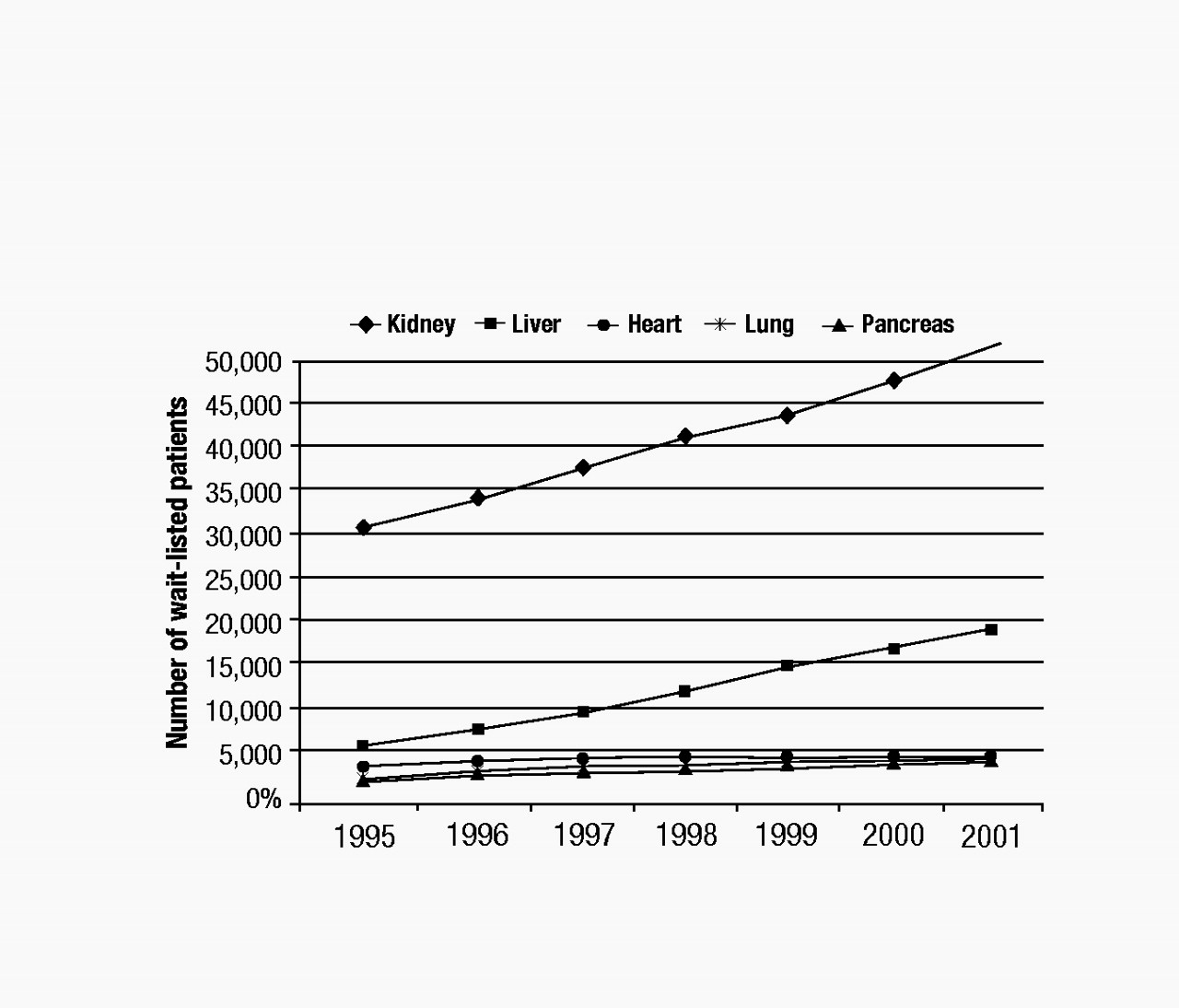
Although immunological barriers still exist for transplant recipients, the greatest obstacle to receiving a transplant is the shortage of donated organs. The number of wait-listed individuals is increasing far beyond the availability of donated organs. As illustrated in Figure 1, the numbers of wait-listed patients for kidney (the most frequent) and liver transplants increased steadily between 1995 and 2001 (
United Network of Organ Sharing 2004). By contrast, the numbers of patients waiting for heart, lung, and pancreas transplants increased only marginally during the same period. The median wait-listed time depends on the organ type, the blood type of the recipient, and the severity of the recipient’s illness at the time of listing. For example, as of 2001, the median wait time for a heart transplant candidate initially listed as a category heart status 2 was 374 days, whereas that for a liver transplant candidate listed as a UNOS 2B was 282 days (
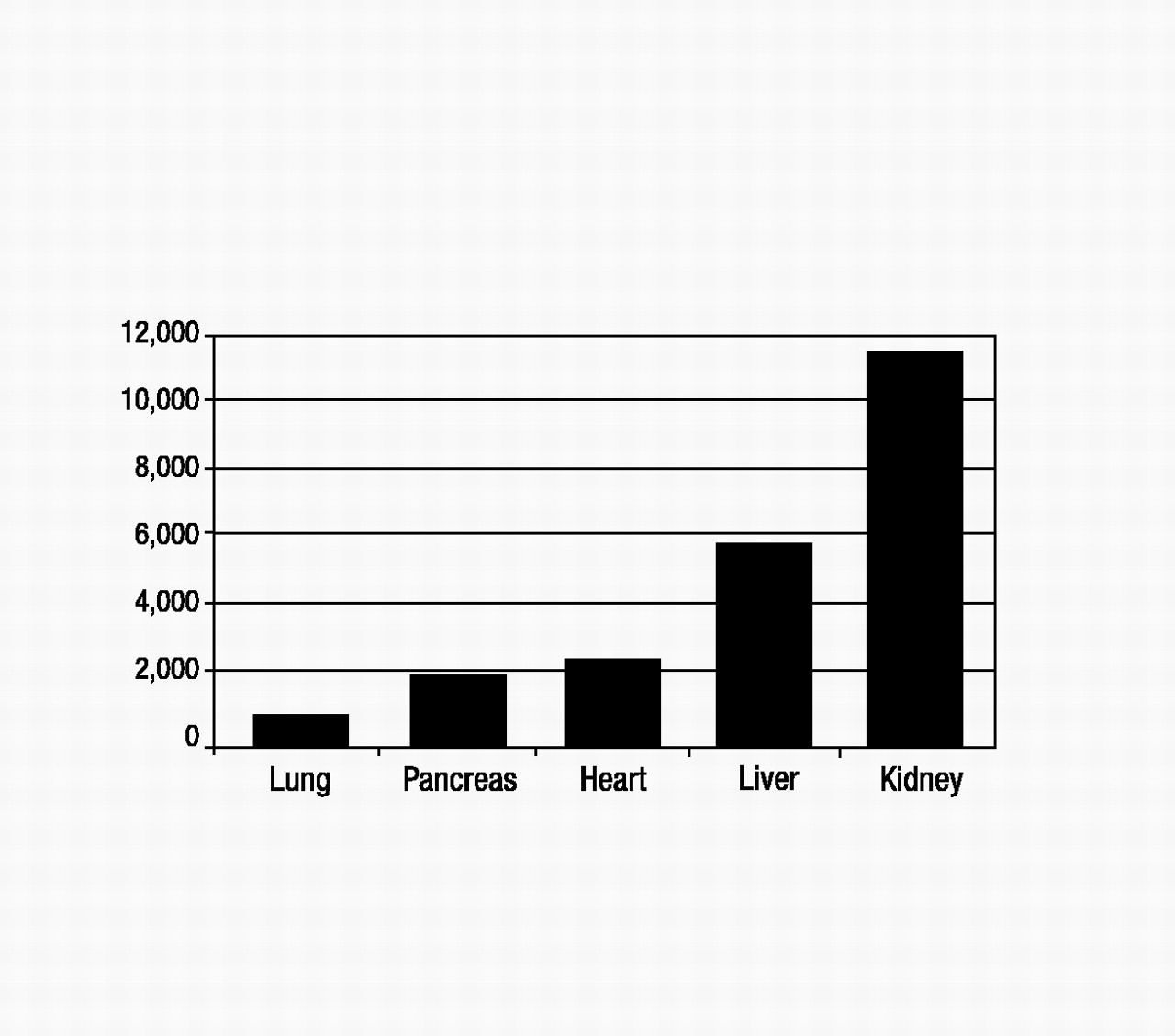
United Network of Organ Sharing 2004). Figure 2 shows the numbers of transplant recipients in 2001 for each solid organ type (
United Network of Organ Sharing 2004), which ranged from a low of 924 for lung to a high of 11,502 for kidney. These numbers are much lower than the 2001 wait-listed values, and ratios of transplant recipients to wait-listed patients are lowest for kidney, liver, and lung (about 1:4 to 1:5). Each year, 10%–15% of liver, heart, and lung transplant candidates will die while on the waiting list (
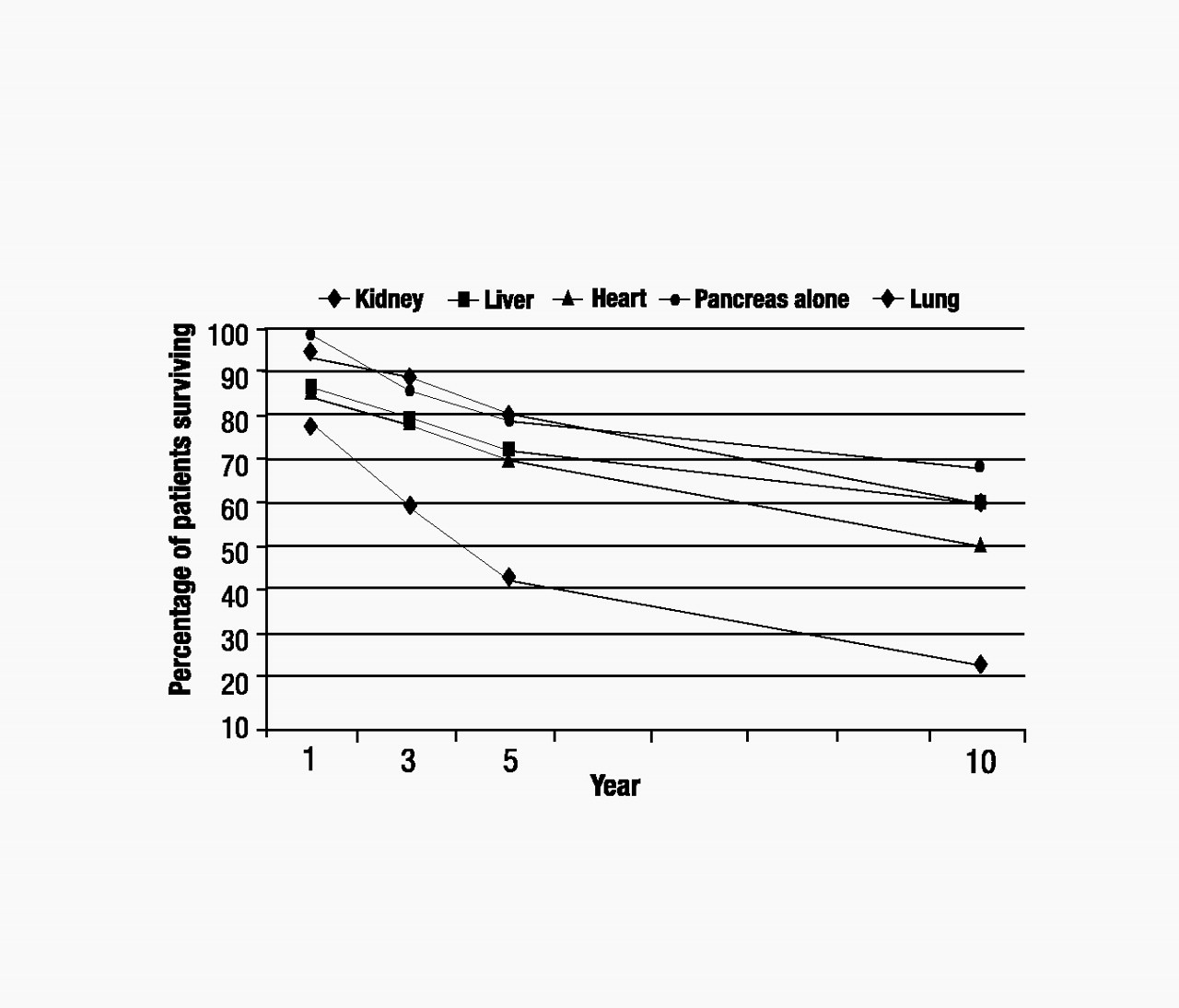
United Network of Organ Sharing 2004). Additionally, posttransplantation graft survival rates can be significantly lower (e.g., 36.4% kidney and 45% liver graft survival after 10 years) than patient survival rates, which means that many transplant recipients will have to face a second transplant 5–10 years after their first (Figure 3) (
United Network of Organ Sharing 2004).
These stark facts highlight the enormous stresses facing transplant candidates, transplant recipients, and their caregivers. These issues have also created a particular environment in which hospitals must evaluate, treat, and select patients for organ transplantation. The scarcity of donated organs has driven efforts to select candidates believed to have the best chance for optimal posttransplant outcomes. Additionally, the organ shortage has increasingly led to the consideration of living kidney donors and, more recently, living liver donors (and, more rarely, living lung donors) as transplantation options.
Pretransplant psychosocial evaluations are commonly requested to assist in candidate and donor selection, and psychiatric consultation is often needed for clinical input during the pre- and posttransplant phases. Although a wide body of knowledge has been developed in the clinical care of transplant candidates and recipients, little longitudinal research is available to answer questions about long-term outcomes or the impact of psychiatric factors (assessed pretransplant and/or in the early years posttransplantation) on outcomes. Research primarily has focused on kidney, heart, and liver transplantation, which in combination currently account for almost 90% of transplants performed in the United States.
In this chapter, we outline the essential areas of the field for psychosomatic medicine specialists and other mental health clinicians involved in the care of transplant patients—pretransplant assessment and candidate selection, emotional and psychological aspects of the transplant process, therapeutic issues, patients with complex or controversial features, psychopharmacological treatment, and neuropsychiatric side effects of immunosuppressive medications. Special pretransplantation topics of emerging importance to psychosomatic medicine specialists are also discussed (i.e., hepatic encephalopathy, ventricular assist devices in heart transplantation, tobacco use, and living donors). The neuropsychiatric sequelae of end-stage organ disease are not covered in this chapter, because those aspects are addressed in the respective chapters on each organ system. Specific transplant issues are also discussed in Chapter 19, “Heart Disease”; Chapter 20, “Lung Disease”; Chapter 22, “Renal Disease”; and Chapter 34, “Pediatrics.”
Pretransplantation issues
Psychosocial/psychiatric assessment
Pretransplant psychosocial evaluations have been a traditional role of the psychiatric consultation team in the transplantation process. These evaluations are frequently used to assist in the determination of a candidate’s eligibility for transplantation, to identify psychiatric/psychosocial problems that may need to be addressed to prepare the candidate and family for transplantation, and to identify pre- and posttransplant psychiatric and/or psychosocial needs of the candidate. These evaluations are also critical for the identification of psychiatric, behavioral, and psychosocial risk factors that may portend poor transplant outcomes (
Crone and Wise 1999;
Dew et al. 2000b).
Transplant programs will often refer for evaluation candidates with a known history of psychiatric problems or those who are identified during the initial clinical interviews with the transplant team as having such problems. Pretransplant psychosocial evaluations are also usually requested for patients with substance use disorders (including tobacco) and other poor health behaviors (e.g., obesity, noncompliance).
Although a truly comprehensive assessment of a potential transplant candidate would require a full psychiatric consultation, the current high numbers of candidates preclude this. To handle the increasing volume of evaluations, some centers employ screening batteries of patient-rated measures to identify candidates with elevated levels of psychological distress, who then undergo a full psychiatric evaluation. Screening instruments can provide baseline cognitive, affective, and psychosocial ratings for candidates; use of these instruments maximizes staff resources and minimizes costs. For example, using this strategy,
Jowsy et al. (2002) identified 20%–44% of liver transplant candidates who had mild to severe symptoms on a range of measures, which prompted a higher level of evaluation.
Emerging evidence shows that preoperatively assessed psychosocial variables can predict posttransplantation psychiatric adjustment among recipients of most organ types (
Dew et al. 2000b). These variables are increasingly being investigated as contributing to medical outcomes as well, although a consistent predictive effect has not yet been demonstrated (
Dew et al. 2000b). Thus, psychosocial assessment of transplant candidates provides an opportunity to identify potential problems and intervene prior to transplantation, with the goal of improving posttransplant outcomes. Transplant programs vary considerably in their psychosocial assessment criteria and procedures (see
Olbrisch and Levenson 1995 for a review of methodological and philosophical issues); in general, however, psychosocial evaluations have 10 objectives (although a given assessment may not include all 10), as enumerated in Table 1 (see
Levenson and Olbrisch 2000).
Because information on all of these domains may not be obtainable during a single clinical interview, a follow-up reassessment may be necessary to clarify relevant issues, solidify a working relationship with the patient and family, and resolve problems. A multidisciplinary approach is often used with input from psychiatrists, psychologists, psychiatric nurse clinical specialists, addiction specialists, social workers, transplant surgeons, and transplant coordinators to construct a comprehensive picture of the patient and develop a coordinated treatment plan. As with any psychiatric evaluation, verbal feedback provided to the patient and family will serve to solidify the expectations of the transplant team and the requirements of the patient for listing if indicated. Some centers also use written “contracts” to formalize these recommendations (
Cupples and Steslowe 2001;
Stowe and Kotz 2001). In difficult cases, these contracts serve to document expectations, thereby minimizing misinterpretation. Written contracts outline a treatment plan that can be referred to with each follow-up appointment. These contracts are particularly useful with transplant candidates who have alcohol or substance abuse/dependence problems, specifying the transplant program’s requirements for addiction treatment, monitoring of compliance (e.g., documented random negative blood alcohol levels), and length of abstinence (see subsection “Alcohol and Other Substance Use Disorders” later in this chapter).
Psychosocial instruments and measures
Transplant-specific (e.g., Psychosocial Assessment of Candidates for Transplant [
Olbrisch et al. 1989], Transplant Evaluation Rating Scale [
Twillman et al. 1993]), disease-specific (e.g., Miller Health Attitude Scale for cardiac disease [
Miller et al. 1981], Quality of Life Questionnaire—Chronic Lung Disease [
Guyatt et al. 1987]), and disorder-specific (e.g., High Risk Alcohol Relapse Scale for alcoholism [
Yates et al. 1993]) instruments have been used to evaluate transplant candidates and monitor their posttransplant recovery. These instruments have been used in conjunction with general instruments for rating behavior, coping, cognitive and affective states, and quality of life. Psychosocial instruments can be used to identify individuals who require further assessment (as described earlier) or to pursue evaluation of patients already identified as requiring additional screening. The evaluator’s purpose for using such instruments will determine the type and specificity of the instruments chosen; for instance, in the subsection “Hepatic Encephalopathy” later in this chapter, we discuss the use of neuropsychiatric tests to aid in the identification of cognitive impairment. Some instruments are more applicable to transplant populations than others. For example, although there are many instruments and measures for assessing alcoholism, none of these instruments are tailored to transplant candidates; they are focused on general issues of detection and treatment of addiction rather than on issues important in evaluating appropriateness for transplantation.
Because psychosocial selection criteria differ significantly by program and organ type, development and use of structured evaluation instruments may help to direct and standardize the transplant selection protocols used nationally. The two instruments most commonly used to assess candidates for transplantation are the Psychosocial Assessment of Candidates for Transplantation and the Transplant Evaluation Rating Scale.
The Psychosocial Assessment of Candidates for Transplantation (PACT) was the first published psychosocial structured instrument specifically designed for screening transplant candidates (
Olbrisch et al. 1989). It provides an overall score and subscale scores for psychological health (psychopathology, risk for psychopathology, stable personality factors), lifestyle factors (healthy lifestyle, ability to sustain change in lifestyle, compliance, drug and alcohol use), social support (support system stability and availability), and patient educability and understanding of the transplant process. The PACT can be completed in only a few minutes by the consultant following the evaluation but requires scoring by a skilled clinician, without which the instrument’s predictive power could be diminished (
Presberg et al. 1995). The final rating for candidate acceptability is made by the clinician, with the freedom to weigh individual item ratings variably (
Presberg et al. 1995). Thus, a single area, such as alcohol abuse, could be assigned greater weight and thus could disproportionately influence the final rating.
The PACT has been used to predict mortality in bone marrow recipients (independent of age, gender, or diagnosis), as well as to predict hospital lengths of stay following liver transplantation (
Levenson et al. 1994). Its “risk for psychopathology” subscale identifies psychopathology that may require referral and treatment after liver, heart, and bone marrow transplantation (
Levenson et al. 1994).
The Transplant Evaluation Rating Scale (TERS) is used to rate patients’ level of adjustment in 10 areas of psychosocial functioning: prior psychiatric history, DSM-III-R Axis I and Axis II diagnoses, substance use/abuse, compliance, health behaviors, quality of family support, prior history of coping, coping with disease and treatment, quality of affect, and mental status (
Twillman et al. 1993). In one study, the TERS was significantly correlated with several clinician-reported outcome variables (compliance, health behaviors, substance use), with particularly high correlations between pretransplant TERS scores and posttransplant substance use (
r=0.64) (
Twillman et al. 1993). The instrument requires administration by a skilled clinician to maintain accuracy (
Presberg et al. 1995). The TERS summary score is derived from a mathematical formula in which individual item scores are multiplied by theoretical, predetermined weightings.
Although individual candidates do not always easily fit within one of the three categories of each item on the TERS, the TERS has more items than the PACT, a feature that may prove useful in future research (
Presberg et al. 1995). However, the PACT is the more flexible of the two instruments, both in the range of rating individual items and in the manner in which the summary score is determined (
Presberg et al. 1995). Together, these instruments are useful in the organization of patient information and can be helpful both as tools for increasing the evaluator’s understanding of the candidate and for research purposes.
The unique role of the psychiatric consultant
Unlike in most psychiatric interviews, the psychiatrist performing the pretransplant assessment primarily serves the needs of the transplant team rather than those of the patient (a possible exception is the evaluation of living organ donors; see subsection “Living Donor Transplantation” later in this chapter). The psychiatric consultant must be candid with the patient about this role. Careful delineation of specific transplant-related expectations, explanation of the importance of these requirements to the success of transplantation, and exploration of the implications of these criteria for the individual candidate serve to establish a meaningful dialogue with the patient from which the therapeutic alliance necessary for future intervention can develop.
For the clinician, the seemingly reverse nature of this role can be uncomfortable or even anxiety provoking. This is especially true if the clinician is not recommending the candidate for transplantation. Fortunately, many programs do not reject patients outright for psychosocial reasons; rather, they offer such patients the opportunity to work to bring their problematic areas into compliance with the recommendations (i.e., through addiction counseling, behavioral changes, psychiatric treatment, identification of appropriate social supports) and then undergo reevaluation for candidacy. In these cases, the psychiatric consultant can often function as an advocate for the patient and assist in referral for appropriate treatment if indicated. Nevertheless, some patients will be unable to comply with the specified transplant requirements or will not survive to complete their efforts to meet candidacy requirements.
Philosophical, moral, ethical, legal, and therapeutic dilemmas are inherent in the role of transplant psychiatrist, as conflicting team opinions present themselves in the course of work with potential transplant candidates. Team discussions and consultation with other colleagues are the rule in complicated cases. In these instances, team discussions not only aid in resolving candidacy quandaries but also can help alleviate team members’ anxiety and discomfort over declining a patient for transplantation. Group or team debriefing may also be desirable, and occasionally consultation with risk management and the legal department of the hospital is needed (e.g., when a candidate is challenging candidacy requirements or the candidacy decision of the transplant team). Thorough documentation is essential in order to delineate the issues involved, the expectations of the team for transplantation candidacy, and the efforts to work with the patient.
Psychological and psychiatric issues in organ transplantation
Psychiatric symptoms and disorders in transplant patients
Similar to other medically ill populations, transplant candidates and recipients experience a significant amount of psychological distress and are at heightened risk of developing psychiatric disorders. The prevalence rates of major depression range from 4% to 28% in liver transplant patients, 0% to 58% in heart transplant patients, and 0.4% to 20% in kidney transplant patients (
Dew 2003;
Dew et al. 2000b). The range of rates for anxiety disorders appears to be 3% to 33% (
Dew 2003;
Dew et al. 2000b), but there are not enough studies to identify specific types of anxiety disorders. One study found that 10% of a cohort of heart or lung transplant recipients experienced posttraumatic stress disorder (PTSD) related to their transplant experience (
Köllner et al. 2002). In a prospective study of 191 heart transplant recipients, the cumulative prevalence rates for psychiatric disorders during the 3 years posttransplantation were 38% for any disorder, including 25% with major depression, 21% with adjustment disorders, and 17% with PTSD (
Dew et al. 2001a). Factors that increased the cumulative risk for psychiatric disorders included a pretransplant psychiatric history, a longer period of hospitalization, female gender, greater impairments in physical functioning, and fewer social supports (
Dew et al. 2001a).
Several studies have suggested an association between psychiatric disorders and transplant health outcomes, although the results have been mixed. A study of wait-listed liver transplant candidates found that candidates with Beck Depression Inventory (BDI) scores higher than 10 (64% of patients) were significantly more likely than nondepressed candidates to die while awaiting transplantation (
Singh et al. 1997). The higher BDI scores were due more to psychological distress than to somatic symptoms. However, for candidates who reached transplantation, pretransplant depression was not associated with poorer posttransplant survival (
Singh et al. 1997). These results were not affected by the severity of and complications from liver disease, or by patients’ social support, employment, or education (
Singh et al. 1997). A study of lung transplant recipients found that those with a pretransplant psychiatric history (anxiety and/or depressive disorders) were more likely than those without such a history to be alive 1 year after transplantation (
Woodman et al. 1999). However, in a study of 191 heart transplant recipients, a DSM-III-R diagnosis of PTSD (with the traumatic event being transplant related) was associated with higher mortality (odds ratio=13.74) (
Dew and Kormos 1999). Another study of heart transplant recipients found that patients with ischemic cardiomyopathy and high self-rated depression scores pretransplant had significantly higher posttransplant mortality compared with the low-depression group after adjustment for sociodemographic and somatic symptoms (
Zipfel et al. 2002). Although causal directions cannot be inferred from these data, studies in other medically ill populations have demonstrated the substantial contribution of depression and anxiety to health outcomes (see Chapter 9, “Depression,” and Chapter 12, “Anxiety Disorders”). Whether treating these disorders will affect patient outcomes is unclear. However, the role of the psychiatrist in evaluating, diagnosing, and treating psychiatric disorders both pre- and posttransplantation is critical.
Adaptation to transplantation
Transplant candidates typically experience a series of adaptive challenges as they proceed through evaluation, waiting, perioperative management, postoperative recuperation, and long-term adaptation to life with a transplant (
Olbrisch et al. 2002). With chronic illness, there can be progressive debility and gradual loss of vitality and of physical and social functioning. Adapting to these changes can elicit anxiety, depression, avoidance, and denial and requires working through of grief (
Olbrisch et al. 2002). Patients who are wait-listed may develop contraindications to transplantation (i.e., infection, serious stroke, progressive organ dysfunction), and both patients and families should be made aware that a candidate’s eligibility can change over time for many reasons (Stevenson 2002). During this phase, psychiatrists may provide counseling to patients and families to help them prepare for either transplantation or death.
The summons for transplantation can evoke a mixture of elation and great fear. Many programs use electronic pagers to contact recipients, and some patients can develop anxiety related to anticipation of the pager’s ring. Patients may experience a panic attack when they are called for transplantation, and some may even decline the offer of an organ.
Much of illness behavior depends on the coping strategies and personality style of the individual. In our experience, the adaptive styles of adult transplant recipients often depend on whether patients’ pretransplant illness experience was chronic or acute, as delineated in the following broadly generalized profiles.
Patients who have dealt with chronic illness for years may adapt psychologically to the sick role and can develop coping strategies that perpetuate a dependency on being ill (
Olbrisch et al. 2002). For these patients, transplantation may psychologically represent a transition from one state of illness to another, and such patients can have difficulty adjusting to or transitioning into a “state of health.” They often complain that the transplant team is expecting too fast a recovery from them, and they may describe feeling pressured to get better. Some patients may develop unexplained chronic pain or other somatic complaints or may begin to evidence noncompliance with transplant team directives.
For patients with good premorbid functioning who become acutely ill, with only a short period of pretransplant infirmity, the transplant can be an unwelcome event. These patients can experience a heightened sense of vulnerability, and they may deny the seriousness of their medical situation (
Olbrisch et al. 2002). These patients often wish to return to normal functioning as quickly as possible posttransplantation, and they may in fact recover more rapidly than the transplant team expects; however, they may suffer later as the result of pushing themselves too much (e.g., returning to work before they are physically ready). They may resent being a transplant recipient, with all of the restrictions and regimens inherent in that role, and may act out their anger or denial in episodes of noncompliance (
Olbrisch et al. 2002).
Treatment modalities
A prospective study of kidney transplant recipients demonstrated that individual psychotherapy was effective in resolving transplant-related emotional problems, with significant reductions in BDI scores after therapy (
Baines et al. 2002). Three recurring psychological themes were expressed by patients in this study: 1) fear of organ rejection, 2) feelings of paradoxical loss after surgery despite successful transplantation, and 3) psychological adaptation to the new kidney (
Baines et al. 2002).
In addition to traditional therapies and pharmacotherapy (see section “Psychopharmacological Treatment in End-Stage Organ Disease” later in this chapter), various innovative strategies have been employed to deal with specific issues of transplantation and also to address logistical and staffing resource issues. At the University of Toronto, a mentoring program was developed for heart transplant recipients. Mentorship by an already transplanted recipient augmented patient care by providing information and support from a peer perspective (
Wright et al. 2001). The four topics most commonly discussed between mentors and mentees were postoperative complications (70%), medications (70%), wait on the transplant list (70%), and the surgery itself (50%) (
Wright et al. 2001). Participants less frequently discussed psychiatric topics such as anxiety (40%) and depression (10%) and personal topics such as sexual relations (20%) and marital problems (10%). The program was well received, and patients were very satisfied with the experience. To increase patient satisfaction with the mentor program, Wright and colleagues recommend early introduction of a mentor and matching of mentors with mentees according to demographics and clinical course (
Wright et al. 2001).
Group therapy for organ transplantation patients and family members has also been successfully used. At the Toronto Hospital Multi-Organ Transplantation Program, group psychotherapy is organized along three dimensions: course of illness (pre- vs. posttransplantation), homogeneous versus heterogeneous group membership (e.g., separate groups for patients and caregivers vs. integrated groups, organ-specific groups vs. cross-organ groups), and group focus (issue-specific vs. unstructured) (
Abbey and Farrow 1998). Increasing levels of group therapy intensity are used, depending on the needs of the patient. Educational groups are mandatory for pretransplant candidates to prepare them for transplantation. From these groups, candidates at risk for psychosocial problems are referred to supportive and psychoeducational groups. Interpersonal and supportive–expressive psychotherapy groups are available to those who require them and have the psychological capacity to benefit from them. Group therapy participants report decreases in negative affect, increases in positive affect and happiness, less illness intrusiveness, and improved quality of life (
Abbey and Farrow 1998). Transplant coordinators also report that patients in group therapy require less contact, both in clinic and by telephone for social support (
Abbey and Farrow 1998).
Dew and colleagues (2004) have developed an innovative strategy for managing the logistical problem of recipients living at a distance from the transplant program. These researchers designed and evaluated an Internet-based psychosocial intervention for heart transplant recipients and their families. This multifaceted Web-based intervention included stress and medical regimen management workshops, monitored discussion groups, access to electronic communication with the transplant team, and information on transplant-related health issues (
Dew et al. 2004). Compared with heart recipients without access to the Web site, intervention patients reported significant reductions in depressive and anxiety symptoms and improved quality of life in the social functioning domain; in addition, caregivers of intervention patients reported significant declines in anxiety and hostility symptoms (
P<0.05). Mental health and quality-of-life benefits were greater among more-frequent users of the Web site. The subgroup using the Web site’s medical regimen workshop showed significantly better compliance at follow-up than did all other patients in attending clinic appointments, completing blood work, and following diet (
Dew et al. 2004). Dew and colleagues concluded that a Web-based intervention could improve follow-up care, compliance, and mental health in patients and families as they adjust to heart transplantation.
Patients with complex or controversial psychosocial and psychiatric issues
The stringency of selection criteria for transplantation appears to depend on the type of organ transplant being considered, and transplant programs often have strongly formed beliefs about the suitability of candidates with certain types of mental illness. Cardiac transplant programs are more likely than liver transplant programs to consider psychosocial issues as contraindications, and liver transplant programs in turn are more stringent than kidney transplant programs (
Corley et al. 1998;
Levenson and Olbrisch 1993). These differences may be attributable to the relative availability of specific types of organs (
Yates et al. 1993); alternatively, the extent of experience with specific organ transplants allowing programs to feel more comfortable with less stringent criteria (e.g., kidney transplantation, with more than three decades of experience and nearly 300,000 kidney transplants performed in the United States) (
United Network of Organ Sharing 2004). In addition, for kidney transplantation, cost-effectiveness research has clearly demonstrated the long-term cost savings of kidney transplantation relative to dialysis (
Eggers 1992). With such unequivocal evidence, insurance payers have a strong financial incentive to refer patients early for preemptive transplantation, before the high costs of dialysis begin to accumulate (
Eggers 1992). In such a setting, psychosocial factors may have less impact on transplantation candidacy. Other issues influencing the selection process include moral and ethical beliefs, societal views, personal beliefs, and even financial constraints.
Although increasing numbers of poor prognostic indicators during the perioperative period may increase risk for noncompliance posttransplantation (
Dew et al. 1996; see subsection “Posttransplant Compliance” below), it should be emphasized that candidates with any one of these features are not categorically poor recipients or that patients without any of these features do not categorically make the best candidates. What little research is available provides some support for clinical assumptions that patients with certain personality disorders, substance use disorders, poor coping skills, poor compliance, and poor social supports can have worse posttransplant outcomes. Nevertheless, case reports have demonstrated that even some patients who might seem inappropriate for transplant (e.g., patients with active psychosis or with severe personality disorders) (
Carlson et al. 2000;
DiMartini and Twillman 1994) can undergo transplantation and maintain adequate compliance after the procedure. Such patients should be carefully assessed pretransplant with optimization of their pretransplant condition and ongoing psychiatric monitoring and treatment posttransplantation. Prospective longitudinal studies are needed to clarify pretransplant factors that contribute to increased risk of poor outcomes (both psychological and medical) and the contribution of posttransplant factors on outcomes as well.
Posttransplant compliance
Lifelong immunosuppression is a prerequisite for good graft function, and noncompliance with immunosuppressive medication is often associated with late acute rejection episodes, chronic rejection, graft loss, and death. It might be assumed that transplant patients, in general, constitute a highly motivated group and that their compliance would be high. Unfortunately, overall posttransplant medical noncompliance rates of all organ types range from 20% to 50% (see
Laederach-Hofmann and Bunzel 2000 for a complete review). With organ transplantation, noncompliance impairs both life quality and life span, as it is a major risk factor for graft-rejection episodes and may be responsible for up to 25% of deaths after the initial recovery period (
Bunzel and Laederach-Hofmann 2000). Noncompliance leads to waste, as it reduces the potential benefits of therapy and adds to the costs of treating avoidable consequent morbidity. Graft loss from noncompliance is also tragic, given the large numbers of patients on the waiting lists. The global assessment of transplant patient compliance is difficult, and patients can manifest varying degrees of adherence to medical recommendations. For transplant recipients, compliance is commonly conceptualized as adherence to immunosuppressive medications. Yet the occurrence of clinically measurable events such as rejection episodes, organ loss, or death underrepresents the true amount of noncompliance, as some patients who are only partially compliant have not yet experienced a clinically adverse event (see
Bunzel and Laederach-Hofmann 2000;
De Geest et al. 1995). Although such “subclinical” noncompliance is undetectable as a medical event, it is important as an indicator of those patients having difficulty following their medical regimens (
Feinstein 1990).
De Geest et al. (1995) reported a medication noncompliance rate of 22.3% in kidney transplant patients, whereas
Paris et al. (1994) found a rate of 47% among heart transplant recipients.
Shapiro et al. (1995) observed that of 93 heart transplant recipients, about one-third (34.4%) were noncompliant in at least some areas over the course of long-term follow-up.
Dew and colleagues (1996) examined compliance in eight domains of posttransplant care in a cohort of 101 heart recipients. During the first postoperative year, the degree of noncompliance varied across time. However, they found persistent noncompliance in the domains of exercise (37%), blood pressure monitoring (34%), immunosuppressive medication (20%), smoking (19%), diet (18%), blood work completion (15%), clinic attendance (9%), and heavy drinking (6%). These studies also identified associations between noncompliance and increased risk of morbidity and mortality in transplant recipients (
De Geest et al. 1995;
Dew and Kormos 1999;
Dew et al. 1996;
Paris et al. 1994).
Dew et al. (1996) carried out a “dose–response” analysis of specific factors found to contribute to noncompliance in a preliminary regression analysis. A “dose” variable was created by determining how many of these six psychosocial risk factors—anxiety, anxiety–hostility, poor support from caregivers, poor support from friends, failure to use active cognitive coping strategies, and use of avoidance coping strategies—a recipient possessed. The logistic regression model showed a strong dose effect, in that if 0 to 1 psychosocial risk factor was present, the probability of having postoperative compliance difficulties was less than 30%. With 2 to 3 factors present, the probability rose to about 50%, and if 4 or more risk factors were present, more than 80% of patients encountered significant compliance difficulties. This means that if predictors cumulate, compliance problems are likely to rise dramatically.
Other studies have determined that psychiatric problems that persist after transplantation are highly associated with noncompliance (
Paris et al. 1994;
Phipps et al. 1997). In an extensive literature review of posttransplant compliance for all organ types,
Bunzel and Laederach-Hofmann (2000) found that anxiety disorders—and, in particular, untreated major depression—were significantly associated with noncompliance. In a study of 125 heart transplant recipients (
Shapiro et al. 1995), compliance problems were associated with a history of substance abuse (
P=0.0007).
Alcohol and other substance use disorders
Compared with other solid organ transplant candidates, liver transplant (LTX) candidates more often require psychiatric consultation for substance addiction assessment, due to the prevalence of alcoholic liver disease (ALD) and viral hepatitis transmitted through contaminated needles. An estimated 50% of LTX recipients have a pre-LTX history of alcohol and/or drug abuse/dependence (
DiMartini et al. 2002). A survey of 69 U.S. liver transplant programs found that 83% of programs have a psychiatrist or addiction medicine specialist routinely see each patient with ALD during the evaluation phase (
Everhart and Beresford 1997). In the optimal situation, the psychiatric clinician is an integral member of the transplant clinical care team and can integrate the addiction treatment plan into the patient’s pre- and posttransplant care. The Cleveland Clinic Foundation has formed a chemical-dependence transplant team to assess, treat, and monitor transplant patients with addictive disorders (
Stowe and Kotz 2001). This program is a model for the integration of such services.
Psychiatric consultation provides a thorough evaluation of the candidate’s addiction history; their understanding of their addiction (especially in the context of their health and need for transplantation, their stability in recovery, and their need for further or ongoing addiction treatment), and the presence of other psychiatric disorders. Family and social support for the candidate’s continued abstinence both pre- and posttransplantation must also be evaluated. In one study of LTX candidates, those with a history of substance abuse revealed significantly more distress, less adaptive coping styles, and more character pathology than their counterparts (
Stilley et al. 1999). Because these features may heighten the potential for relapse, periodic reassessment by the psychiatric consultant provides follow-up on the candidate’s progress in recovery, including verifying ongoing participation in rehabilitation as well as monitoring for psychological and affective distress and poor coping styles with therapeutic interventions targeting these problems as they arise. Documentation of treatment participation is desirable, as is random toxicological screening for alcohol and other substances. These measures are especially important for patients early in recovery and for those with a short period of abstinence, denial over their problem, resistance to seeking treatment, or poor social support for continuing abstinence. One study of pretransplant wait-listed ALD candidates found that 15% of candidates had used alcohol at some point after the initial transplant evaluation (
Weinrieb 2003).
One-year post-LTX drinking rates (i.e., the percentage who used any alcohol by 1 year post-LTX) range from 8% to 37% (
DiMartini 2000b;
Everson et al. 1997), with cumulative rates estimated at 30%–40% by 5 years post-LTX (
Lucey 1999). Rates of pathological drinking, defined as drinking that results in physical injury or alcohol dependence, are 10%–15% (
Everson et al. 1997;
Fireman 2000). Although the rate of alcohol use appears to attenuate with the passage of time, post-LTX (
Berlakovich et al. 1994;
Campbell et al. 1993), dependent drinking can occur years post-LTX (
DiMartini 2000a). In one study, 15% of LTX recipients had their first drink within the first 6 months post-LTX, a finding that highlights the importance of early and intensive clinical follow-up to identify alcohol use at its onset (
DiMartini et al. 2001).
Consistent predictors of posttransplant alcohol use have been difficult to identify. This may be due to the heterogeneity of the ALD transplant population and the potential selection bias whereby the most stable candidates are chosen, making this population different from the general alcohol-abusing/dependent populations (
DiMartini et al. 2002). For example, a pretransplant history of illicit drug use has not been consistently associated with increased risk for posttransplant alcohol relapse in ALD recipients (
Coffman et al. 1997;
DiMartini et al. 2002;
Fireman 2000;
Foster et al. 1997;
Newton 1999), possibly because many ALD recipients had discontinued their drug use many years prior to transplantation (
Coffman et al. 1997). In one of the few prospective studies to examine posttransplant alcohol use, a pretransplant history of alcohol dependence, a family history of alcoholism, and prior rehabilitation experience (thought to be a marker for those with more severe addiction) were all found to be associated with posttransplant alcohol use (
P<0.05). A prior history of other substance use was associated with a higher (but non–statistically significant) risk of posttransplant drinking (
DiMartini 2000b).
Compared with LTX candidates with alcohol dependence, LTX candidates with polysubstance dependence are more likely to have multiple prior addiction treatments; more likely to be diagnosed with personality disorders, especially cluster B type (antisocial, narcissistic, histrionic, borderline); and less likely to have stable housing, a consistent work history, or stable social support (
Fireman 2000). Yet despite evidence that this specific population could be at higher risk for relapse, there are few published outcome studies addressing the issue of posttransplant nonalcohol substance use. Most studies have investigated the rates of relapse only in ALD recipients who also had a nonalcohol other substance use disorder. One of the few studies to investigate all patients with a pre-LTX addiction history found not only that patients with a pre-LTX history of polysubstance use disorders had a higher relapse rate compared with those with alcohol dependence alone (38% vs. 20%), but also that the majority of polysubstance users demonstrated ongoing post-LTX substance use (
Fireman 2000). Studies investigating all transplant recipients are needed to identify the true posttransplant rates of other substance use.
After transplantation, maintaining an open, nonjudgmental dialogue with transplant recipients appears to be the most effective way to identify alcohol and/or other substance use in the posttransplant period, and most recipients are open to discussing their substance use habits with the transplant team (
DiMartini et al. 2001;
Weinrieb et al. 2000). A review of liver enzymes and biopsy results and a candid discussion of the damage caused by alcohol and other substances provide an opportunity to explore the patient’s denial of the consequences of their use. Even in the most difficult cases, patients wish to maintain their health and are willing to listen to advice and recommendations on addiction treatment. In our experience, the transplant team has established a powerful emotional bond with the recipient. Many patients who have resumed substance use were relieved to learn that the transplant team would not abandon them. On the other hand, it is important not to condone or dismiss small amounts of alcohol or other substance use. What may seem supportive can be distorted by the patient with an addiction and become an excuse to use more regularly. In the case of alcohol use, we have found that few patients with alcoholism can drink “socially” posttransplantation (
Tringali et al. 1996) and that those who take their first drink often consume moderate to heavy amounts of alcohol (
DiMartini et al. 2002). Therefore, total alcohol abstinence is recommended for these patients.
Medications that may reduce cravings and potentially diminish relapse risk for alcohol (e.g., acamprosate, ondansetron, naltrexone) or opioids (naltrexone) have not been studied in transplant patients. One study that attempted to use naltrexone in actively alcohol-relapsing LTX recipients found that patients were reluctant to use naltrexone as a result of its potential, albeit small, risk of hepatotoxicity (
Weinrieb et al. 2001). Naltrexone can be a direct hepatotoxin at dosages higher than recommended (>300 mg/day) and is not recommended for patients with active hepatitis or liver failure. Disulfiram has been used in nontransplant populations to provide a negative reinforcement to drinking alcohol. This agent blocks the oxidation of alcohol at the acetaldehyde stage and can create severe nausea, vomiting, and hemodynamic instability. It requires hepatic metabolism for conversion into an active drug. A metabolite of disulfiram is an inhibitor of cytochrome P450 3A4 (
Madan et al. 1998), and posttransplantation may cause immunosuppressive medication toxicity. Use of disulfiram in transplant recipients could place these individuals at risk for serious harm and is not recommended. In nontransplant patients, selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) can stabilize mood and improve abstinence rates in depressed relapsing alcoholic individuals (
Cornelius et al. 2003) and may be the most appropriate pharmacological interventions for transplant recipients if concurrent mood symptoms are present.
Methadone-maintained candidates
Transplant program acceptance of opioid-dependent patients receiving methadone maintenance treatment (MMT) is a controversial issue. Recent studies have examined candidate selection processes and posttransplant outcomes for this population.
In a recent survey of U.S. liver transplant programs (
Koch and Banys 2001), of the 56% of programs that reported accepting patients for evaluation who were taking methadone, a surprising 32% required patients to discontinue their methadone use prior to transplantation. Of even more concern was the overall lack of experience with such patients (i.e., only 10% of the programs had treated more than five MMT patients). Although there are no studies of pretransplant methadone cessation in liver transplant patients, there exists an abundance of evidence showing that tapering methadone in stable methadone-maintained patients results in relapse to illicit opiate use in up to 82% of these individuals (
Ball and Ross 1991). In our opinion, an attempt to taper a recovering opiate addict from methadone should not be made at a time when the patient is struggling with the stresses and pain associated with end-stage liver disease. Until data to the contrary emerge, requiring methadone tapering in stable opiate-dependent patients as a prerequisite for transplant candidacy could be considered unethical. This strategy potentially heightens the risk for relapse, and those that relapse would be denied transplantation.
In regard to posttransplant outcomes of MMT patients,
Koch and Banys (2001) found that of the approximately 180 transplant patients on methadone maintenance at the time of the survey, relapse to illicit opiate use was reported for less than 10% of patients. Similar to other reports of noncompliance in transplant patients (see subsection “Posttransplant Compliance” earlier in this chapter), approximately 26% of MMT patients had compliance difficulties with immunosuppressive medications (
Koch and Banys 2001). However, it was not reported whether those who used illicit drugs were also among those who had problems with compliance. In general, the transplant programs did not consider that the noncompliance necessarily affected outcomes, and the transplant coordinator’s impressions were that only 7 of 180 patients had poor outcomes (
Koch and Banys 2001). In two small series of MMT LTX recipients (5 in each), overall long-term patient and graft survival were found to be comparable to those of other LTX recipients at the transplant centers, with none of the MMT patients evidencing posttransplant noncompliance or illicit drug use (
Hails and Kanchana 2000;
Kanchana et al. 2002).
Liu et al. (2003), in a study of the largest single cohort (
N=36) of MMT LTX recipients to date, concluded that patient and graft survival were comparable to national averages (they did not use a control group, however). Although four patients (11%) reported isolated episodes of heroin use posttransplantation, relapses were not considered to have resulted in poorer outcomes.
These descriptive studies demonstrate that MMT transplant recipients can successfully undergo transplantation and do well; however, the lack of control groups makes it difficult to prove that MMT patients’ rates of complications and survival are no different from those of transplant recipients in general. Although the small numbers of MMT candidates and recipients preclude large-scale prospective studies, a case–control study conducted by
Gordon et al. (1986) found that posttransplant patient and graft survival rates in the 20 heroin-addicted kidney recipients were similar to those in the control group. The leading cause of death (infection) was the same in both groups. In the study group, only one patient returned to heroin use. Two other study patients who were not suspected of using heroin lost their grafts as a result of medication noncompliance, whereas no patient in the control group lost a graft as a result of noncompliance (
Gordon et al. 1986). In summary, the data to date justify neither automatic exclusion of MMT patients from transplantation nor any requirement that such patients be tapered off methadone prior to transplant.
Personality disorders
Personality disorders are characterized by persisting and inflexible maladaptive patterns of subjective experience and behavior that may create emotional distress and interfere with the individual’s interpersonal relationships and social functioning. The requirements of successful transplantation can be too difficult for such an individual, as the process requires a series of adaptations to changes in physical and social functioning and significant ability to work constructively with both caregivers and the transplant team. By identifying personality traits and disorders, the psychiatrist can potentially predict patterns of behavior, recommend treatment, develop a behavioral plan with the team to work constructively with the patient, and render an opinion as to the candidate’s ability to proceed with transplantation. Patients with personality disorders can require excessive amounts of time from the transplant team, which raises the issue of resource allocation as a potential selection criterion (
Carlson et al. 2000). Not surprisingly, a majority of programs (50%–60% across organ types) consider personality disorders to be a relative contraindication to transplantation (
Levenson and Olbrisch 1993). Yet all personality disorders should not be viewed similarly, as the behavioral and coping styles of different personality disorders can present varying degrees of concordance with the needs of transplantation. For example, the need for structure and orderliness of a candidate with obsessive-compulsive personality disorder would be more adaptive to the demands of transplantation than the coping style of a patient with borderline personality disorder.
The incidence of personality disorders in transplant populations is similar to that in the general population, ranging from 10% to 26% (
Chacko et al. 1996;
Dobbels et al. 2000), although in some cohorts estimates have been as high as 57% (
Stilley et al. 1997). However, the identification of personality disorders depends on the definition and measurement methods used. Unfortunately, studies investigating personality disorders and transplantation outcomes have not distinguished among the various personality disorder types (perhaps because of the low prevalence of each type), which makes generalizations difficult. Nevertheless, case reports of patients with severe character pathology demonstrate the extent of compliance problems that can arise from these disorders, resulting in significant morbidity and recipient death (
Surman and Purtilo 1992;
Weitzner et al. 1999). The disturbances in interpersonal relationships that can occur with personality disorders also can decrease the likelihood that patients will have stable and reliable social supports during the pre- and posttransplant phases (
Yates et al. 1998). Of the personality disorders, borderline personality disorder is considered to represent the highest risk for posttransplant noncompliance (
Bunzel and Laederach-Hofmann 2000).
Whereas sociopathy has not consistently been associated with substance relapse in the addiction literature (
Vaillant 1997), a survey of transplant programs in the United States revealed that 4 of 14 programs (29%) would reject a candidate with comorbid antisocial personality disorder and alcohol dependence (
Snyder et al. 1996). In a study of 73 ALD transplant candidates, patients with severe personality disorders had higher rates of divorce, higher rates of comorbid drug abuse/dependence, lower IQs, higher scores on indicators of emotional impairment, and were more likely, although not significantly so, to return to drug use during the pretransplant follow-up period (
Yates et al. 1998). However, of this cohort, 3 patients with serious personality disorders underwent liver transplantation and did not relapse or become noncompliant in the early postoperative phase (
Yates et al. 1998). In contrast, another study of 91 patients transplanted for ALD and followed for up to 3 years identified 18 patients exhibiting antisocial behavior (
Coffman et al. 1997). Of those with antisocial behavior, 50% returned to either alcohol (
n=6) or prescription narcotic addiction (
n=3) posttransplantation, which was significantly higher than the 19.8% alcohol use by the total group (
Coffman et al. 1997). In a prospective study of 125 heart transplant recipients, personality disorders were associated with posttransplant compliance problems (
P=0.007) (
Shapiro et al. 1995). Although personality disorders were not associated with survival, those individuals with personality disorders tended to have more graft rejection (
P=0.06) (
Shapiro et al. 1995).
Although not identified as specific personality disorder styles, various coping and behavioral styles have also been shown to influence survival. A study by
Chacko et al. (1996) of survival post–heart transplant found that whereas the presence of clinician-rated Axis II disorders (26% of the group) was not associated with survival time, some of the strongest predictors of survival were health behaviors, maladjustment, and coping styles. Using the same measure of health behavior and coping (Millon Behavioral Health Inventory [
Millon et al. 1982]),
Coffman and Brandwin (1999) found that wait-listed heart transplant candidates with high scores on the Life Threat Reactivity subscale had significantly higher mortality before transplantation (42% vs. 18%;
P=0.0001) but not after transplantation. These investigators suggested that one possible explanation was that the detrimental psychological traits of the high-risk group were ameliorated by surviving to be transplanted (
Coffman and Brandwin 1999).
Patients with personality disorders do best with ongoing pre- and posttransplant psychotherapy, specifically cognitive and behavioral interventions to promote compliance with the care regimen and to establish a working alliance with transplant team members (
Dobbels et al. 2000). These patients should be given clear and consistent instructions on rules and requirements of transplantation, reinforced by regular outpatient appointments. A limited number of transplant center staff should maintain contact with the patient, and staff should communicate regularly among themselves and the outpatient psychiatric team (
Carlson et al. 2000) to coordinate care and to reduce opportunities for cognitive distortions and splitting by the patient. A formal written contract can document the expectations of the transplant team and serve as a therapeutic treatment plan whereby the patient and team agree to work together toward common goals for the transplant recipient’s health (
Dobbels et al. 2000).
Psychotic disorders
Although chronic and active psychosis is thought by many to be incompatible with successful transplantation, case reports of carefully selected patients with psychosis demonstrate that such patients can successfully undergo transplantation and survive after the procedure (
DiMartini and Twillman 1994;
Krahn et al. 1998). A recent survey of transplant psychiatrists at national and international transplant programs identified only 35 cases of pretransplant psychotic disorders in transplant recipients from 12 transplant centers (
Coffman and Crone 2002), suggesting that such patients are highly underrepresented among transplant recipients. Results of this survey confirmed previously expressed stipulations that patients with psychotic disorders be carefully screened before acceptance. Candidates should have demonstrated good compliance with both medical and psychiatric follow-up; possess adequate social supports, especially in-residence support; and be capable of establishing a working relationship with the transplant team. In this survey (
Coffman and Crone 2002), risk factors for problems with compliance after transplantation included antisocial or borderline personality disorder features, a history of assault, living alone, positive psychotic symptoms, and a family history of schizophrenia. Posttransplant noncompliance with nonpsychiatric medications was found in 20% of patients (7 of 35), and noncompliance with laboratory tests was found in 17% (6 of 35 patients) (
Coffman and Crone 2002); however, these numbers are similar to percentages of medication and laboratory testing noncompliance in general transplant populations. Overall, noncompliance resulted in rejection episodes in 5 patients (14%) and in reduced graft function or loss in 4 patients (12%) (
Coffman and Crone 2002). Thirty-seven percent of patients experienced psychotic or manic episodes posttransplantation (not necessarily associated with immunosuppression), 20% attempted suicide (with two completed suicides), 20% experienced severe depression or catatonia, 5.7% committed assaults, 5.7% were arrested for disorderly conduct, and 8.6% required psychiatric commitment (
Coffman and Crone 2002).
Although concerns have been raised in regard to the potential of immunosuppressive medications to produce or exacerbate psychotic symptoms, patients with a prior psychiatric history are not necessarily more susceptible to “steroid psychosis” than are patients without such a history (
Hall et al. 1979), and appropriate use of antipsychotic medication is usually adequate to manage these symptoms if they emerge. Because transplant teams often overlook the early postoperative reinstitution of antipsychotic medications, it is essential that the psychiatrist devote careful attention to this issue during the immediate postoperative phase. If quick reintegration of the patient into his or her pretransplant outpatient psychiatric treatment regimen is not possible because of infirmity, interim in-home psychiatric follow-up care should be instituted.
Special issues during the pretransplant phase
Hepatic encephalopathy
Hepatic encephalopathy (HE), a neuropsychiatric syndrome commonly encountered in liver transplant candidates, is characterized by a constellation of signs and symptoms, such as alteration of consciousness (including stupor or coma), cognitive impairment, confusion/disorientation, affective/emotional dysregulation, psychosis, behavioral disturbances, bioregulatory disturbances, and physical signs such as asterixis. Identification of HE is important, because its symptoms directly affect patient quality and quantity of life; fulminant HE is associated with intracranial hypertension, cerebral edema, and death pretransplant (
Ferenci et al. 2002). It is also critical to differentiate between the fluctuating course of HE and more persistent cognitive deficits that may indicate a preexisting dementia rather than delirium. Recent efforts to define HE have emphasized that it reflects a continuum of symptoms, with subclinical HE lying at the minimal end of the spectrum. Even subclinical HE is clinically important, because it can impair patient safety (
Schomerus et al. 1981) and is associated with persistent cognitive deficits post-LTX (
Tarter et al. 1990). In one study, 85% of patients with subclinical HE were found to be either of questionable fitness or unfit to drive on psychometric testing of driving capacity (
Schomerus et al. 1981). By definition, subclinical HE is not identifiable on a typical clinical examination; detection may require additional neuropsychological tests of psychomotor speed, praxis, concentration, and attention. The Trail Making Test (A and B) and the Digit Symbol and Block Design tests from the Wechsler Adult Intelligence Scale—Revised are commonly used to identify subclinical HE impairment. Whereas the prognostic significance of HE is well known, the long-term impact of subclinical encephalopathy on cognitive functioning requires further investigation.
The predominant strategy for treating HE involves reducing the production and absorption of ammonia from the intestinal tract, although a variety of compounds and metabolites (e.g., mercaptans, false neurotransmitters, manganese, endogenous benzodiazepines, increased concentrations of central nervous system [CNS] gamma-aminobutyric acid [GABA]) have also been implicated in HE (
Chung and Podolsky 2003;
Riordan and Williams 1997). Psychiatric consultants should be familiar with ammonia-reducing strategies, because they are often the ones who must recognize and monitor HE symptoms, identify whether patients are being treated for HE, and make recommendations regarding the need for initiating or improving treatment. HE can be precipitated by gastrointestinal hemorrhage, uremia, use of some psychoactive medications or diuretics, dietary indiscretions, dehydration, or electrolyte imbalance (
Chung and Podolsky 2003;
Riordan and Williams 1997), and these problems should be corrected first. Treatment should strive to normalize ammonia levels, despite the fact that blood ammonia levels are not well correlated with symptoms of HE (
Riordan and Williams 1997). Treatment strategies include administration of a nonabsorbable disaccharide, lactulose, that acts as an osmotic laxative to flush out ammonia; adherence to a protein-restricted diet to decrease the production of ammonia from protein; and prescription of nonabsorbable antibiotics to reduce intestinal bacteria that convert protein to ammonia. Some patients require all three treatments simultaneously. Medications that can contribute to symptoms of HE—anticholinergic drugs, tranquilizers, and sedatives—should be avoided.
Ventricular assist devices in heart transplantation
Progress in the development of implantable left ventricular assist devices (LVADs) has dramatically improved both the physical and the psychological health of potential cardiac transplant candidates. The new LVADs consist of a mechanical pump implanted in the abdomen with conduits from the apex of the left ventricle to the ascending aorta. Blood returning from the lungs to the left side of the heart exits through the left ventricular apex into the LVAD pumping chamber. Blood is then actively pumped through the LVAD outflow valve into the ascending aorta. One transcutaneous line carries an electrical cable to an external battery pack and electronic controls, which are worn on a shoulder holster or belt.
Prior to the use of LVADs, the need for prolonged inotrope infusions before transplantation could lead to very lengthy hospitalizations for cardiac transplant candidates. These patients would become deconditioned and were at risk for deep vein thrombosis, pulmonary emboli, multiple organ dysfunction, and sudden cardiac death. However, recent experience with newer LVADs reveals improvement in mechanical/electrical failure rates and lessened risks for thromboembolism (
Rose et al. 2001a,
2001b). Patients on LVADs can achieve better hepatic, renal, cerebral, and peripheral perfusion, leading to improvement in overall physical function, exercise tolerance, and well-being (
Goldstein et al. 1998;
Morrone et al. 1996). These devices are now portable, permitting discharge from the hospital before transplantation and a generally acceptable quality of life (
Dew et al. 2000a;
Frazier 1993;
Loisance et al. 1994). Patients on LVADs can undergo physical and physiological rehabilitation, develop exercise tolerance, and rebuild muscle mass, thus stabilizing their cardiac condition (
Goldstein et al. 1998;
McCarthy 2002;
Morrone et al. 1996). The lack of mobility restriction means that patients often can return to work and engage in activities such as dancing and driving (
Catanese et al. 1996). With the urgency for transplantation diminished, the transplant team can wait for an optimal donor organ.
On the downside, however, the logistics of arranging outpatient care require a well-trained medical team whose members are available at all times, resulting in significant patient, caregiver, and medical system burden. All persons involved in the patient’s care must receive extensive training, arrangements for outpatient housing or maintenance at home must be coordinated, and local emergency paramedical personnel must also be trained. Infection can occur in more than 60% of LVAD recipients (
Gordon et al. 2001); in one study of patients with permanent LVADs, 41% of deaths were related to infection (
Rose et al. 2001b). Not surprisingly, in a study of recipients of ventricular assist devices (including both LVADs and biventricular assist devices), the most common concern was risk of infection (52%) (
Dew et al. 2000a). In addition, 52% had difficulty sleeping because of the driveline, 46% had pain at the drive-line site, 40% worried about device malfunction, and 32% were bothered by the noise (
Dew et al. 2000a). In posttransplantation comparisons with heart recipients who did not receive a ventricular assist device (VAD), patients who were bridged to transplantation with a VAD showed similar improvements in physical functioning and emotional well-being, significantly lower rates of anxiety, but poorer cognitive status (
Dew et al. 2001b). The cognitive impairments observed in the VAD recipients were believed to be attributable to neurological events that occurred during the period of VAD support and were higher than that of non-VAD patients during the waiting period before transplantation. Although mild, these impairments appeared to persist during the first year following transplantation and were associated with less likelihood of returning to employment (
Dew et al. 2001b). In the future, as the technology continues to improve, selected patients may receive LVADs as permanent implants rather than as bridges to transplantation (
Rose et al. 2001a,
2001b).
Tobacco use and transplantation
Tobacco use by transplant candidates and recipients has received surprisingly little attention. Even in lung and heart transplantation, tobacco use is not routinely reported. Tobacco use coupled with immunosuppressive therapy, which also increases cancer risk (
Nabel 1999), may result in higher rates of cancer posttransplantation. For ALD liver transplant recipients in one study, the rates of oropharyngeal cancer and lung cancer were 25 and 3.7 times higher, respectively, than rates in the general nontransplant population matched for age and gender (
Jain et al. 2000), presumably as a result of tobacco use.
One study of heart transplant recipients found that 26%–50% of smokers resumed smoking posttransplantation (
Bell and Van Triget 1991;
Nagele et al. 1997). Compared with nonsmokers, smokers had higher rates of vasculopathy and of malignancies (
Nagele et al. 1997); they also had significantly worse survival, with none of the smokers surviving 11.5 years posttransplantation (vs. 80% of the nonsmokers surviving). When patients were grouped by carboxy-hemoglobin level, investigators found that no patients with a level higher than 2.5% were surviving 4 years after transplantation (
Nagele et al. 1997). In this cohort, smoking appeared to be much more important than other classical risk factors (
Nagele et al. 1997). Similarly, a study of liver transplant recipients found a higher rate of vascular complications in patients with a history of smoking (17.8% vs. 8% in patients without such a history;
P=0.02); furthermore, having quit smoking 2 years prior to transplantation reduced the incidence of vascular complications by 58% (
Pungpapong et al. 2002). In a prospective study of ALD liver transplant recipients, 53% were found to be using tobacco posttransplantation (
DiMartini et al. 2002). A study of 60 heart transplant recipients reported that 3 patients had resumed smoking within 6 months following transplantation and that all 3 had also relapsed to drug or alcohol abuse (
Paris et al. 1994). Another study of heart transplant recipients found that elevated posttransplant anxiety was associated with a higher risk of resuming smoking (
Dew et al. 1996).
The cessation of tobacco use (both smoked and smokeless) prior to transplantation is strongly recommended, given that many pretransplant users resume use posttransplantation. Treatments for smoking cessation include bupropion, nicotine replacement (patches, gum, lozenges, and aerosolized formulations), and behavioral therapies (
Hurt et al. 1997;
Jorenby et al. 1999). Because of its association with seizure risk, bupropion should be used with caution in transplant recipients, who are already at increased risk from immunosuppressive medications, particularly during the early posttransplant period, when immunosuppressive levels are higher. A good alternative would be nortriptyline, which in combination with cognitive-behavioral therapy in general-population studies has been demonstrated to have a smoking cessation success rate similar to that of bupropion (
Hall et al. 1998). Notwithstanding, the anticholinergic side effects of nortriptyline could be problematic in candidates or recipients at risk for delirium, and although the drug’s α-adrenergic, antiarrhythmic, and negative inotropic effects are tolerated post–heart transplant (
Kay et al. 1991;
Shapiro 1991), these effects should be carefully monitored in heart transplant candidates.
Living donor transplantation
Despite the physical risks, discomfort and pain, expense and inconvenience, and potential psychological consequences of donating an organ, increasing numbers of people are becoming donors, and transplant programs are considering living donation as one solution to the organ shortage. In fact, in 2001 the number of living donors exceeded the number of cadaveric donors (6,526 vs. 6,081) for the first time, with the majority from kidney donors, although an increasing number are coming from living liver donors in the form of a partial hepatectomy (
United Network of Organ Sharing 2004). Currently, more than 50% of kidney transplants come from living donors (
United Network of Organ Sharing 2004). Kidneys and portions of the liver, lung, pancreas, intestine, and even the heart (through a domino procedure in which a heart-lung recipient donates their heart) are donated for transplantation (
Oaks et al. 1994;
Rodrigue et al. 2001;
Taguchi and Suita 2002).
Donation of an organ—putting one’s life at risk to help another—is an incredibly generous and altruistic gift. Yet the evaluation of such donors is complex process requiring assessment of the circumstances and motives of the donor, the dynamics of the relationship between donor and recipient, the severity of the recipient’s illness, and family and societal forces. Current practice guidelines require a psychosocial evaluation for each potential donor to thoroughly examine these and other issues (Table 2) (
Olbrisch et al. 2001;
Surman 2002). Donors must be fully willing, independently motivated, and completely informed about the surgery. Yet for liver donors in particular, long-term sequelae that may affect the donor’s future health, functioning, and even ability to obtain health insurance (due to the presence of a preexisting condition) are not known.
Living liver donation is a much more surgically complex, invasive, and potentially more dangerous procedure than kidney donation. Although mortality rates have been less than 1% for both kidney and liver donors (
Brown et al. 2003;
Najarian et al. 1992), about one-third of liver donors have complications, with serious complications occurring in 14% of donors (
Brown et al. 2003;
Grewal et al. 1998). There have been consensus recommendations that all potential live liver donors be evaluated by an independent physician advocate (i.e., not a member of the transplant team responsible for the recipient’s care) as part of the informed consent process (
Abecassis et al. 2000;
Conti et al. 2002) to avoid conflicts of interest. However, in only 50% of programs does a physician who is not part of the transplant team evaluate the potential donor (
Brown et al. 2003). Kidney donors should expect to miss 4–6 weeks of work and liver donors 8–12 weeks of work, especially if the job involves heavy lifting. Since the late 1990s, laparoscopic donor nephrectomy has been increasingly used, a procedure that results in less postoperative pain, shorter hospital stays, overall quicker recovery times, and more favorable cosmetic results. Future research may also show this approach has psychosocial benefits as well.
Adult-to-adult living donor liver transplantation (LDLT) is a relatively new procedure in the United States, preceded by adult-to-child transplants. Whereas only 9 such procedures had been performed in the United States prior to 1998, in 2001 more than 400 adult-to-adult LDLTs were performed. Unfortunately, too few procedures are performed at any one center—and approaches to recipients and donors are too diverse across centers—to provide reliable and generalizable information about donor and recipient outcomes. In one study that examined outcomes of parent-to-child liver donation, psychological testing was found to be useful in identifying families that were more likely to experience problems postdonation (
Goldman 1993). Although donor outcomes were reported as good, with donors experiencing increased self-esteem and satisfaction, marital dissolution occurred in 2 of the 20 families following donation (
Goldman 1993). In another study, one donor committed suicide 2 years after donation, and although this event was deemed by the transplantation center to be unrelated to the donation, the details were not known (
Brown et al. 2003).
A U.S. live organ donor consensus group recommended the development of a living donor registry to collect demographic, clinical, and outcome information on all living organ donors. The rationale for the development of such a registry includes concern for donor wellbeing, limitations of current knowledge regarding the long-term consequences of donation, the potential to evaluate the impact of changes in criteria for donor eligibility on the outcome of donors, and the need within the transplant community to develop mechanisms to provide for quality assurance assessments (
Abecassis et al. 2000). In the near future, a multicenter study of adult-to-adult living liver donor outcomes commissioned by the National Institute of Diabetes and Digestive and Kidney Diseases should help provide answers to these questions (see
“Adult-to-Adult Living Donor Liver Transplantation Cohort Study [A2ALL]” 2003).
In contrast with the United States, Japan has extensive experience with living liver donation as a result of cultural beliefs (lack of acceptance of brain death criteria) that hamper cadaveric donation. This created an environment in which living liver donation was necessary for LTX. More than 1,500 adult-to-adult LDLTs have been performed in Japan, with no reported donor mortality (
Surman 2002).
Fukunishi et al. (2001) first reported on psychiatric outcomes in LDLT donors and recipients, identifying post-LTX psychiatric disorders (excluding delirium) in 37% of LDLT recipients and “paradoxical reactions” (including guilt over receiving donation, avoidant coping behaviors, and psychological distress) in 34% of recipients, despite favorable medical outcomes for both recipient and donor. Ten percent of liver donors experienced major depression within the first month after donation. Fukunishi and colleagues speculated that predonation, the stronger sense of duty of adult children donating to their parents masked their true concerns and fears. Following donation, these concerns manifested as anxiety, fear, and pain (
Fukunishi et al. 2003). The prevalence of psychiatric disorders was higher in LDLT recipients than in a comparison group of living donor kidney recipients, suggesting a potential greater need for psychiatric evaluation and care of LDLT patients.
Altruistic donors—those donating to an unknown recipient—pose one of the most complex challenges to transplant evaluation. In these cases, the psychosocial evaluation has particular importance in determining the suitability of the donor, and some believe that the medical standards for such donors should be higher (
Friedman 2002). Altruistic donors are commonly viewed with some skepticism and are evaluated with greater caution than related donors. A detailed evaluation is critical, both to understand the motives and psychological meaning of the donation to the donor and to identify any financial or other types of compensation expected for the donation. A recent study of nondirected donors reported that 21% were excluded for psychological reasons (
Matas et al. 2000). Psychological outcomes of altruistic liver donors are unknown.
Psychopharmacology in end-stage organ disease
Given the high prevalence of psychiatric disorders in transplant candidates and recipients, pharmacological treatment is often required. Unfortunately, psychotropic medications are often not provided because of concerns about patients’ medical fragility and the potential risks of psychotropic medications. The fact that psychiatric symptoms may evolve from complex, intertwined psychological and physiological processes should not preclude treatment. Although the treatment of transplant candidates and recipients can be complicated, a thorough knowledge of psychotropic pharmacokinetics in specific types of end-stage organ failure, coupled with careful attention to medication dosing, side effects, and drug interactions, can provide the necessary foundation for pharmacological management (see also Chapter 37, “Psychopharmacology”;
Robinson and Levenson 2001;
Trzepacz et al. 2000).
Liver disease
Liver failure affects many key steps of medication pharmacokinetics, from absorption to metabolism, distribution, and elimination, causing changes in drug levels, duration of action, and efficacy. As the liver becomes cirrhotic, collateral blood vessels develop that circumvent the liver. Intrahepatic and extrahepatic physiological (and, in many patients, surgical) shunts create portal–systemic diversion that reduces liver perfusion and particularly affects first-pass metabolism as less drug is delivered to hepatic enzymes. Loss of functional parenchyma and hepatic enzymes decreases phase I metabolism (such as the cytochrome P450 enzyme system) or phase II metabolism (conjugation enzymes). Most psychotropic medications require hepatic metabolism and are highly bound to plasma proteins. The liver’s production of plasma proteins is reduced, with a resulting increase of the free and pharmacologically active fraction of highly protein-bound drugs. These processes will raise effective drug levels, increasing the risk of drug side effects or toxicity (
Leipzig 1990;
Levy 1990;
Pond and Tozer 1984). Increased volume of distribution resulting from fluid retention (ascites, peripheral edema) can lower effective levels of both water-soluble and highly protein-bound drugs (because more will be in the unbound state due to hypoproteinemia) (
Klotz 1976).
The etiology and severity of liver disease will determine changes in drug pharmacokinetics, including changes in enzymatic activity that affect drug metabolism. In addition, the type of disease (e.g., cholestatic vs. noncholestatic liver disease), the type of disease process (e.g., periportal [affecting phase II enzymes] vs. pericentral [affecting phase I cytochrome P450 enzymes] disease processes), and the type of drug (high-clearance or flow-dependent vs. low-clearance or enzyme-saturating drugs) all play an important role in pharmacokinetics.
One semiquantitative measure of liver functioning, the Child-Pugh Score (CPS), uses commonly available clinical and biochemical indices to rate the severity of end-stage liver disease. The CPS provides a readily reproducible and standardized method for assessing the degree of liver failure and is generalizable to patients with liver disease regardless of its etiology (
Albers et al. 1989). Although the categories of the CPS may overgeneralize the complexities of drug pharmacokinetics, the indices used identify impairment in pharmacokinetic functions (i.e., protein production, portal–systemic shunting, ascites), making the CPS total rating a useful guideline for psychotropic medication dosing (see Table 3). Nevertheless, for the newer medications the pharmaceutical companies typically publish detailed information on drug absorption, metabolism, and elimination, with suggested guidelines for patients with hepatic disease (often using the CPS), and these documents should be referred to for specific medication dosing. In our clinical experience, we have found that patients rated as having CPS class A liver failure are early in the disease process and can usually tolerate 75%–100% of a standard dosage. Those with CPS class B should be dosed more cautiously, starting with a 50%–75% reduction in the normal starting dose. As a result of the prolongation of the elimination half-life and the subsequent delay in reaching steady state, more gradual increments in dosing are required. Patients with CPS class B liver failure can often obtain relief or remission of symptoms with 50% of a typical psychotropic medication dosage. Those with CPS class C commonly have some degree of hepatic encephalopathy, and medication usage must be cautiously monitored to avoid worsening of the hepatic encephalopathy symptoms. Sometimes distinguishing whether depressive symptoms represent primary depression or the affective dysregulation of hepatic encephalopathy may not be possible, in which case a cautious empirical trial of medication may be warranted.
Renal disease
Reduced renal clearance of drugs can occur in renal insufficiency due to primary renal disease or from hypoperfusion in hepatorenal syndrome (
McLean and Morgan 1991) or severe heart failure (
Shammas and Dickstein 1988). Reduced renal clearance is especially important for psychotropic medications that are predominantly excreted by the kidneys (e.g., lithium, gabapentin, topiramate, methylphenidate) and also for psychotropic drugs for which renal excretion of active metabolites is the primary route of elimination (e.g., venlafaxine and its active metabolite
O-desmethylvenlafaxine). In uremia, drug oxidation is normal or accelerated, reduction and hydrolysis are slowed, glucuronide and glycine conjugations are normal and acetylation may be slowed (
Reidenberg 1977). Excess urea may cause gastric alkalinizing effects, decreasing intestinal absorption of some medications (
Levy 1990). In renal failure, hypoalbuminemia, coupled with the inhibition of drug–protein binding from uremia and the accumulation of endogenous protein-binding inhibitors (
Wilkinson 1983), can increase free drug concentrations, especially for drugs with protein binding greater than 80% (i.e., most psychotropics). The effect of diminished renal clearance was demonstrated in a study of TCAs in which inactive metabolites reached 500% to 1,500% of normal levels (
Lieberman et al. 1985). Although these metabolites are thought to be clinically inert, they can alter disposition of the active drug by displacing it from protein binding, competing for active transport mechanisms, and inhibiting drug metabolism (
Lieberman et al. 1985). For patients in severe renal failure, dosing can be complicated (see Chapter 37, “Psychopharmacology”), and renally excreted psychotropic medications should be used very cautiously.
Heart disease
In cardiac insufficiency and congestive heart failure, organ hypoperfusion (especially the liver and kidneys) and increased volume of distribution of drugs due to “third spacing” into interstitial tissues result in decreased drug metabolism and clearance (
Shammas and Dickstein 1988). In one study of patients with congestive heart failure, the half-life of midazolam was prolonged by 50% and the plasma clearance was reduced by 32% (
Patel et al. 1990). Right-sided heart failure can lead to intestinal and hepatic venous congestion, which can impair drug absorption and metabolism, respectively (
Shammas and Dickstein 1988). Acute hypoxia can change blood flow dynamics. Splanchnic blood flow is reduced in the liver, decreasing metabolism, and renal blood flow is also reduced (
du Souich and Erill 1978). Hepatically cleared drugs are more affected than renally cleared drugs in congestive heart failure (
Woosley 1987). Patients with severe right-sided heart failure with passive congestion of the liver should be treated as patients with hepatic insufficiency and dosages initially reduced by 50%.
Lung disease
In pulmonary disease, the binding of some drugs to plasma proteins may be increased, resulting in a lengthened time course of the drug effect, increased drug distribution, and more rapid hepatic and renal clearance (
du Souich and Erill 1978). As with heart disease, acute hypoxia can reduce splanchnic blood flow to the liver, decreasing drug metabolism, and can reduce renal blood flow (
du Souich and Erill 1978). Increased pulmonary vascular resistance, leading to decreased cardiac output (cor pulmonale), causes increased systemic venous pressures, which in turn decrease perfusion of the liver and kidneys. The lungs also contain cytochrome P450 isoenzymes, which may play a minor role in first-pass and overall drug metabolism (
Pond and Tozer 1984). Additionally, high-affinity binding sites for both imipramine and SSRIs have been identified in the lungs, perhaps reflecting the high affinity of these drugs for the serotonin transporter (
Suhara et al. 1998). The significance of these findings in heart and lung disease patients is unknown, although it is speculated that the lungs act as a reservoir for medications with a high affinity to the serotonin transporter (
Suhara et al. 1998).
Neuropsychiatric side effects of immunosuppressive agents
Recent advances in our understanding of immunology and the development of newer strategies for immunosuppression may significantly reduce the need for—if not obviate completely—long-term maintenance immunosuppression. In the future, transplant recipients of all organ types may require immunosuppressive medication dosages only one or two times a week, or not at all (
Starzl 2002). This achievement would remove the final obstacle to long-term successful outcomes for transplant recipients, given that the majority of long-term morbidity and mortality is due to chronic immunosuppression (e.g., infections, renal failure, cancer). Additionally, reduced requirements for immunosuppressive medication would aid in medication compliance and relieve some of the financial burden of long-term immunosuppression. However, for now, transplant recipients will continue to require immunosuppressive therapy and to be subject to their potential neurotoxic and neuropsychiatric side effects. Psychiatrists should be familiar with the signs, symptoms, differential diagnosis, neuroimaging findings, and management of immunosuppressive neurotoxicity and secondary psychiatric disorders in solid organ recipients (
Strouse et al. 1998).
Cyclosporine
Cyclosporine (Gengraf, Neoral, Sandimmune), a lipophilic polypeptide derived from the fungus
Tolypocladium inflatum Goma, is used as a primary immunosuppressive agent. Side effects are usually mild and include tremor, restlessness, and headache (
Wijdicks et al. 1999). A smaller proportion of patients (12%) experience more serious neurotoxicity characterized by acute confusional states, psychosis, seizures, speech apraxia, cortical blindness, and coma (
deGroen 1987;
Wijdicks et al. 1995,
1996;
Wilson et al. 1988). A higher incidence (33%) of serious neurotoxic side effects was reported in one study of 52 liver transplant recipients. Seizures were experienced by 25%; less commonly reported effects included central pontine myelinolysis, delirium, cerebral abscess, and psychosis (
Adams et al. 1987).
More recent evidence suggests that earlier reports of serious neurological side effects may have been attributable to intravenous administration and higher dosages (
Wijdicks et al. 1999). The use of the oral form of cyclosporine (Neoral) results in fewer serious neurological side effects (
Wijdicks et al. 1999). Cyclosporine trough levels correlate poorly with cyclosporine neurotoxicity (
Wijdicks et al. 1999), although in most studies symptoms resolved when the cyclosporine was discontinued and subsequently reinstated at a lower dosage (
Wijdicks et al. 1999). Anticonvulsants can successfully treat cyclosporine-induced seizures but are not required long-term (
Wijdicks et al. 1996), and seizures may cease with reduction or discontinuation of cyclosporine. A few patients with serious clinical neurotoxic side effects have been found to have diffuse white matter abnormalities, predominantly in the occipitoparietal region, on computed tomography (CT) scanning (
deGroen et al. 1987;
Gijtenbeek et al. 1999;
Wijdicks et al. 1995). In one case, symptoms of cyclosporine-induced cortical blindness resolved with drug discontinuation, although pathological evidence of CNS demyelination persisted for months afterward (
Wilson et al. 1988).
Several mechanisms may contribute to the CNS neurotoxicity of cyclosporine. Hypocholesterolemia has been found in a high percentage of patients with serious neurotoxicity (
deGroen et al. 1987;
Wijdicks et al. 1995). Access may be particularly high in the white matter, with a relatively high density of low-density lipoprotein receptors (
Wijdicks et al. 1995). In addition to hypocholesterolemia, hypertension, hypomagnesemia, and the vasoactive agent endothelin may play a role in the pathogenesis of cyclosporine neurotoxicity (
Gijtenbeek et al. 1999).
Tacrolimus
Tacrolimus (FK506, Prograft), a macrolide produced by
Streptomyces tsukubaensis, is used as primary immunosuppressive therapy, as rescue therapy for patients who fail to respond to cyclosporine, and as treatment for graft-versus-host disease. It is more potent and possibly less toxic than cyclosporine, although the neuropsychiatric side effects appear to be similar (
DiMartini et al. 1991;
Freise et al. 1991). As with cyclosporine, neuropsychiatric side effects are more common with intravenous administration and diminish with oral administration and dosage reduction. Common symptoms include tremulousness, headache, restlessness, insomnia, vivid dreams, hyperesthesias, anxiety, and agitation (
Fung et al. 1991). Cognitive impairment, coma, seizures, dysarthria, and delirium occur less often (8.4%) and are associated with higher plasma levels (
DiMartini et al. 1997;
Fung et al. 1991). FK506 can produce symptoms of akathisia (
Bernstein and Daviss 1992). However, a prospective study of 25 renal transplant recipients found no correlation between FK506 plasma levels and scores on an akathisia rating scale, although higher plasma levels were associated with higher levels of subjective restlessness, tension, and autonomic and cognitive symptoms of anxiety (
DiMartini et al. 1996).
FK506 has low aqueous solubility and cannot be detected in the cerebrospinal fluid of patients with suspected neurotoxicity (
Venkataramanan et al. 1991). However, because FK506 has been identified in the brain tissue of animals, it is believed to cross the blood–brain barrier in humans. In addition, more serious neurotoxic side effects (focal neurological abnormalities, speech disturbances, hemiplegia, and cortical blindness) may occur from higher CNS levels in patients who have a disrupted blood–brain barrier (
Eidelman et al. 1991). In a study of 294 consecutive transplant recipients on FK506, those with preexisting CNS damage (e.g., from stroke, multiple sclerosis) were at higher risk for neurotoxic side effects (
Eidelman et al. 1991). A rare syndrome of immunosuppression-induced leukoencephalopathy, involving demyelination (particularly in the parieto-occipital region and centrum semiovale), has been described in transplant recipients receiving FK506 (
Small et al. 1996). The clinical presentation includes generalized seizures without a clear metabolic etiology and radiographic abnormalities in the cerebral white matter of the parietal/occipital lobes. Like other serious neurotoxic side effects, this syndrome is not associated with the absolute serum level of FK506 but does resolve on discontinuation of FK506 (
Small et al. 1996). The mechanism of FK506 neurotoxicity is unclear but may include direct activity at the CNS neuronal level (
Dawson and Dawson 1994) or an immune-mediated cause (
Wilson et al. 1994).
Corticosteroids
Although chronic corticosteroid use has become less essential in immunosuppression for most patients posttransplantation, high dosages of corticosteroids are still employed in the early postoperative phase and also as “pulsed” dosages to treat acute rejection. Behavioral and psychiatric side effects are common, but conclusions regarding the incidence or characteristics of these effects—or the specific dosages required to cause such effects—are not well established. Serious psychiatric side effects have a reported incidence of 5%–6% (
Kershner and Wang-Cheng 1989;
Lewis and Smith 1983) and include a wide range of cognitive, affective, psychotic, and behavioral symptoms (
Hall et al. 1979;
Kershner and Wang-Cheng 1989;
Lewis and Smith 1983;
Varney et al. 1984). These side effects are reviewed elsewhere in
The American Psychiatric Publishing Textbook of Psychosomatic Medicine, particularly in Chapter 9, “Depression”; Chapter 11, “Mania, Catatonia, and Psychosis”; Chapter 23, “Endocrine and Metabolic Disorders”; and Chapter 25, “Rheumatology.”
Sirolimus
Sirolimus (SRL, rapamycin, Rapamune), a macrocyclic lactone isolated from
Streptomyces hygroscopicus, is a recent addition to the posttransplant immunosuppressive armamentarium. The side-effect profile of sirolimus so far does not include neurotoxicity (
Watson et al. 1999), perhaps because sirolimus does not block calcineurin (
Sindhi et al. 2001). However, a systematic evaluation of sirolimus neurotoxicity has yet to be conducted.
Azathioprine
Azathioprine (Imuran) is a purine analog first used in organ transplantation in 1968. It is primarily used as an adjunctive immunosuppressive agent and is less widely used today because of the availability of alternative agents. Specific neuropsychiatric side effects have not been reported for this agent, although CNS complications of immunosuppression are a mechanism to keep in mind. Several reports of depressive symptoms in patients receiving azathioprine have been complicated by the concurrent use of other medications (specifically cyclosporine and prednisone) that may have contributed to mood disturbance. Nevertheless, caution is recommended when using azathioprine in patients with a history of depression.
Mycophenolate mofetil
Few neuropsychiatric symptoms have been reported with mycophenolate mofetil (CellCept), a relatively new immunosuppressant promoted as an improvement over azathioprine. Adverse CNS events (>3% to <20% incidence) included anxiety, depression, delirium, seizures, agitation, hypertonia, paresthesias, neuropathy, psychosis, and somnolence (
Roche Pharmaceuticals 2003); however, because the patients in whom these symptoms occurred were being treated with mycophenolate in combination with cyclosporine and corticosteroids, the precise contribution of mycophenolate to the symptoms is difficult to interpret.
Drug interactions between psychotropic and immunosuppressive medications
Many of the immunosuppressive medications (i.e., tacrolimus, cyclosporine, and mycophenolate) are metabolized by cytochrome P450 3A4; thus, concurrent use of psychotropic medications that strongly inhibit 3A4 should be avoided. Specific cytochrome P450 3A4 inhibitors capable of interacting adversely with immunosuppressive medications, in decreasing order of inhibition, are as follows: fluvoxamine, nefazodone > fluoxetine > sertraline, TCAs, paroxetine > venlafaxine (see also Table 37–1 in Chapter 37, “Psychopharmacology”). There are case reports in which nefazodone has caused toxic tacrolimus levels (
Campo et al. 1998) and a 70% increase in the trough plasma level of cyclosporine (
Helms-Smith et al. 1996). In a study in which fluoxetine and TCAs were used to treat depressed transplant recipients, no difference in cyclosporine blood level–to–dosage ratios and dose–response relationships was found between those treated and those not treated with antidepressants (
Strouse et al. 1996). This finding suggests that antidepressants with less cytochrome P450 3A4 inhibition may not have clinically meaningful drug interactions with these immunosuppressive medications.
Psychotropic medications theoretically may alter immune function. Some psychotropic medications induce a variety of neuroendocrine and cellular actions that could have immunological effects, yet this area has not been well investigated (
Surman 1993). For example, lithium has been shown to enhance neutrophil migration, increase phagocytosis by macrophages, and amplify mitogen stimulation of lymphocytes (
Surman 1993). Although the role of these effects in allograft function is not known, lithium has been used to increase the number of peripheral neutrophils (
Ballin et al. 1998) to reduce neutropenia. The modulatory effects of psychotropic drugs on the immune system are largely unexplored but may provide valuable information for the future care of transplant patients (see
Kradin and Surman 2000 for a complete review).