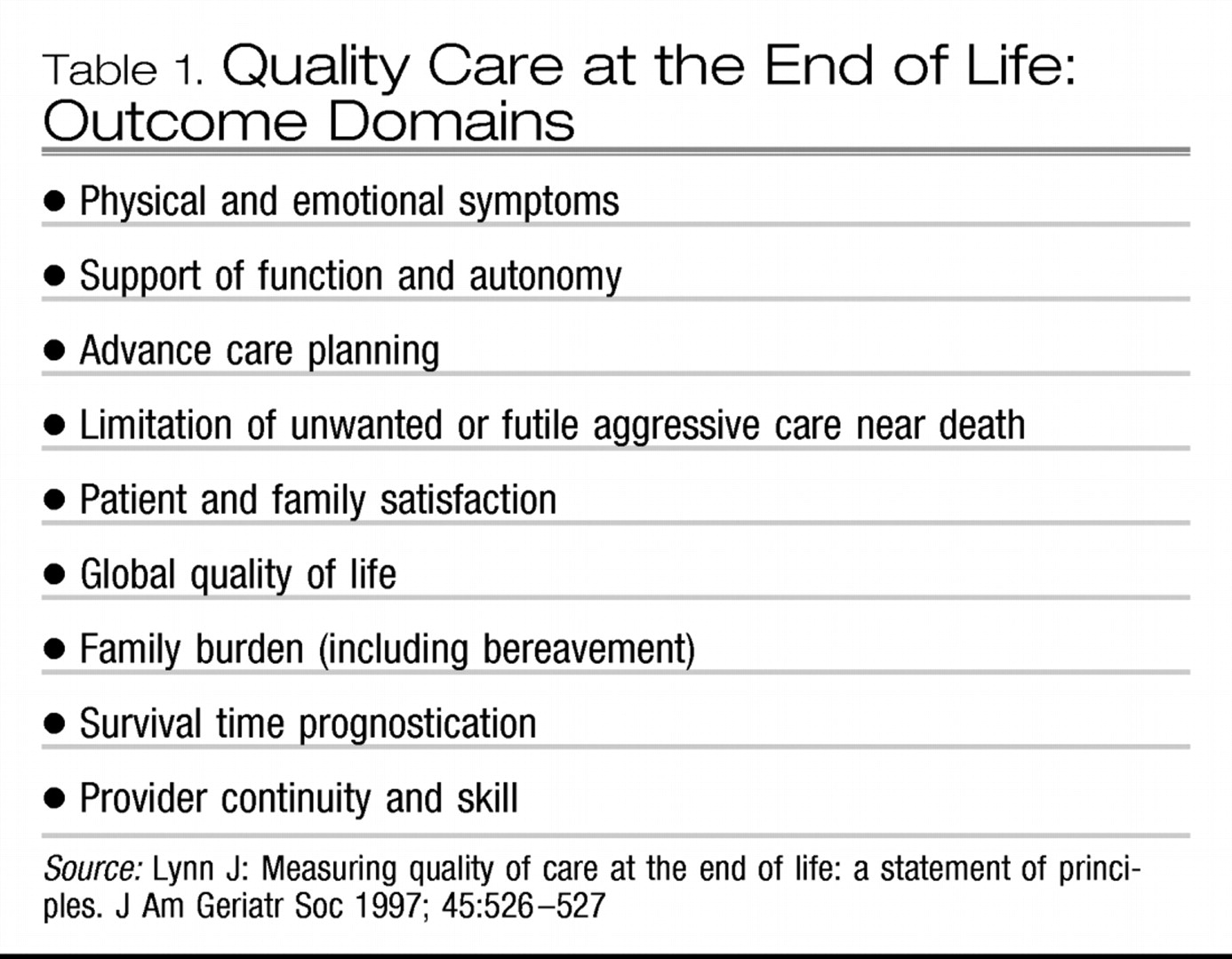
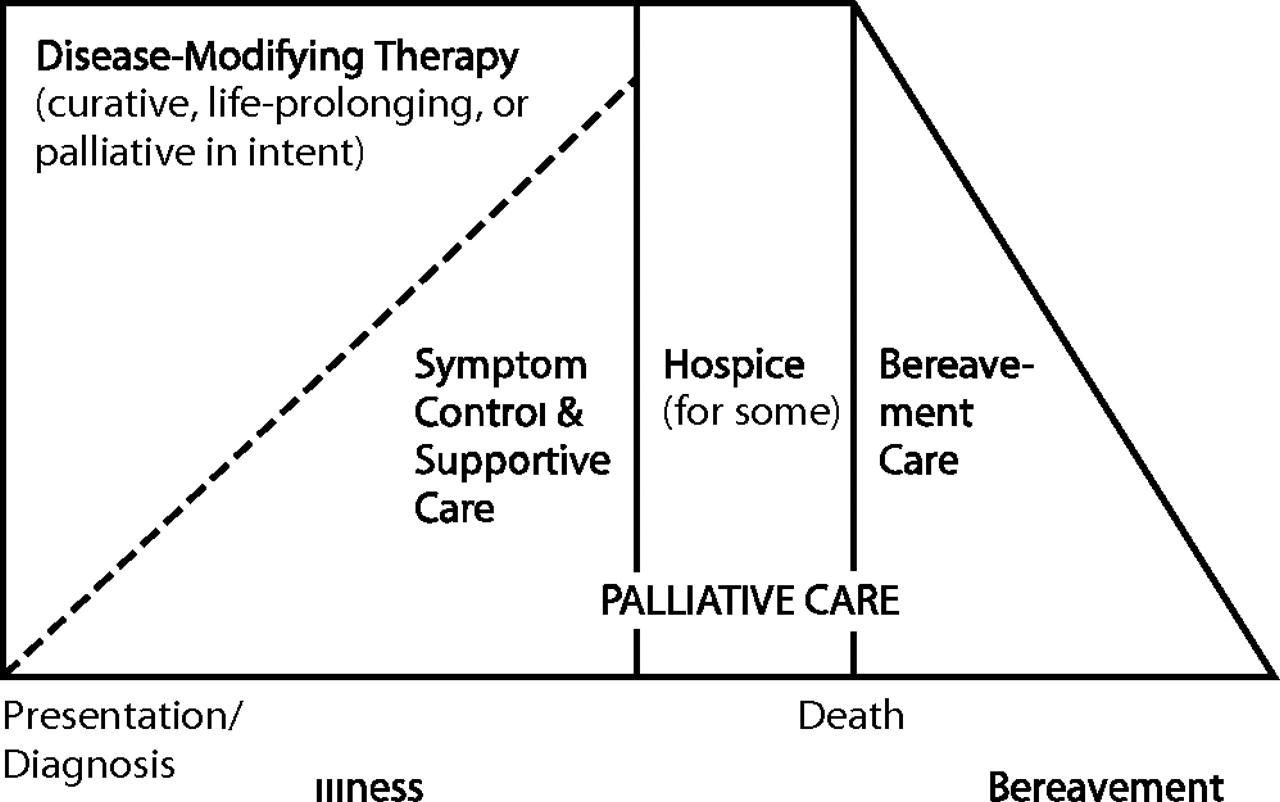
Distressing symptoms in dying patients may occur as part of dysfunction in any organ system. Some may represent underlying medical emergencies, to which decisions must be made as to the level of interventions attempted. Common symptoms most relevant to psychiatry include the following:
Pain.
Physical pain is among the most common symptoms in dying patients, and among the most feared by patients and families. The control of pain is a crucial topic, one that requires more space and has received it elsewhere (
40–
43). Geriatric psychiatrists, in common with colleagues in consultation psychiatry as well as other subspecialties, should have expertise in the assessment and management of pain. Some basic tenets of pain control are listed here.
1. The goal is to eliminate pain. For most patients, it is possible to achieve this goal fully, or at the least to reduce pain to tolerable levels without intolerable side effects. There are some patients who do not want to have their pain eliminated. In this event, careful discussion should elicit whether this wish arises out of, for example, (actual or feared) side effects of analgesics, depression-related hopelessness, or the patient's religious or other cultural values precluding acceptance of pain treatment; in the latter case, exploration with the patient and family can determine whether there is any flexibility within the value/belief system.
2. Pain must be routinely assessed. This is best done by direct inquiry, asking the patient to rate pain on a scale of 0–10 (or by using an equivalent visual-analog scale when verbal communication is difficult); such inquiries may be included in routine nursing assessments as the “fifth vital sign.” It must be recognized that there is no substitute for such direct inquiries of pain ratings by the patient. Observer ratings frequently under-rate pain, partly because chronic pain may manifest as depression, irritability, or other nonspecific symptoms rather than as the visible discomfort we expect to see in acute pain. Because depression so commonly exists concurrently with pain, and may color perceptions of the pain or treatment options, depression must be assessed as well; see below.
3. In many cases, optimal pain treatment may include “disease-focused” therapies, whether or not the use of such therapies can be expected to improve survival or overall disease progression. Common examples include anti-anginal drugs for angina pain, radiation therapy for bony carcinomatous metastases, or glucocorticoids to reduce pain associated with inflammation and edema.
4. Analgesic drugs are a mainstay of pain control. A systematic approach to drug choice should be applied, using a fixed-dose regimen to provide around-the-clock relief along with prn (or “rescue”) medications for break-through pain. The regimen should be reevaluated frequently and increased (in frequency or dosage) if break-through pain is present more than rarely. The World Health Organization analgesic ladder should be used to choose drug class on the basis of severity of pain (
41,
44). Options for Step 1, mild pain, include acetaminophen and nonsteroidal antiinflammatory drugs (including aspirin and the COX-2 inhibitors). For Step 2, moderate pain, opioids such as oxycodone or codeine are given, typically in combination with a Step 1 drug. For Step 3, severe pain, opioids such as morphine are the treatment of choice. Route of administration will depend on the drug, the patient's ability to swallow or absorb an oral dose, and convenience of access to other routes (e.g., nasogastric or direct enteral access, permanent intravenous ports). For some drugs and clinical situations, continuous administration, for example, a subcutaneous morphine drip, with patient control of the dose may be most appropriate.
Other drugs may provide analgesia for certain types of pain. For severe bone pain due to metastatic cancer, co-administration of non-steroidal anti-inflammatory drugs (NSAIDs) along with opioids is often effective (
45–
47). There is some empirical evidence to support the use of other adjuvant drugs in refractory bone pain; these include corticosteroids, biphosphonates, and calcitonin (
48–
52). For neuropathic pain syndromes refractory to opioids alone or co-administered with NSAIDs, empirical evidence best supports the use of tricyclic antidepressants (
53,
54). There is limited evidence for the efficacy of selective serotonin reuptake inhibitors (SSRIs), and even more limited data for other antidepressants, including venlafaxine, bupropion, and mirtazapine (
54–
58). The use of anticonvulsants is commonly recommended as well, although the empirical evidence for indications other than trigeminal neuralgia, and for agents other than carbamazepine, is limited at best (
59). Many other agents have been recommended for neuropathic pain, each with very limited evidence, especially as applied to terminal-illness contexts (
60–
65).
Side effects may limit the tolerability of analgesics. Common problems with opioids include nausea, constipation or obstipation, and sedation or other neuropsychiatric symptoms (e.g., perceptual disturbances with or without the presence of full-fledged delirium). Sometimes switching to a different opioid allows achieving adequate analgesia with fewer side effects in a particular patient. Combination of opioids with other classes of analgesics may allow reduction in opioid dosage with still-adequate pain control. In some cases, additional drugs may be used successfully to counteract opioid side effects, for example, the common prophylactic use of laxatives to prevent uncomfortable constipation or the use of psychostimulants to reduce opioid-induced sedation (
66,
67).
Non-pharmacological approaches to pain may provide benefit in selected individuals and are widely used, although the empirical evidence for their efficacy varies widely, depending on the condition and the modality (
68,
69). These approaches range from invasive (e.g., anesthetic or surgical interventions) to noninvasive physical modalities (e.g., physical rehabilitation approaches, transcutaneous electrical nerve stimulation (TENS), acupuncture, massage) to cognitive/interpersonal approaches (e.g., meditation, guided imagery, relaxation techniques). Geriatric psychiatrists may use their skills in such psychotherapeutic options as guided by patient preferences.
Depression.
It is well recognized that depressive symptoms and syndromes have clinically important bi-directional relationships with comorbid medical conditions. Indeed, medical illnesses are among the most consistently identified correlates of the presence and course of depression in later life, while, conversely, depression is a powerful predictor of functional outcomes and mortality in broad populations, as well as in many specific chronic medical diseases (
70,
71). The clinician or researcher seeking to diagnose depression in dying patients may face confounds in symptom assessment familiar to geriatric psychiatrists, who have expertise working with depression in medically ill patients—namely, how to “count” neurovegetative symptoms (e.g., anergia, anorexia, weight loss, sleep or psychomotor disturbance) toward the syndromic diagnosis of depression, when such symptoms may be due to underlying medical disease even in the absence of depression. Although DSM-IV asks clinicians to take an “etiologic” approach in practice, it is often difficult to judge whether the medical disease or depression causes a particular symptom. Other diagnostic approaches have been described, including exclusive (i.e., eliminating potentially confounded neurovegetative symptoms), substitutive (replacing confounded neurovegetative symptoms with additional emotional or ideational symptoms), or inclusive (counting all symptoms toward the depressive syndrome, regardless of potential etiological confound) (
72). For research purposes, a multi-institutional work group recommended the inclusive approach, based on reliability considerations as well as prevalence and prognostic validity studies that fail to confer a clear advantage for one approach over another (
73). However, many clinicians working with dying patients continue to recommend an essentially exclusive approach, that is, an emphasis on the emotional and ideational symptoms of depression (
74). Even assessments of the latter symptoms are difficult in palliative care contexts, because it is not clear how to operationally define some ideational symptoms, such as hopelessness or recurrent thoughts of death, in persons facing impending death. Moreover, the conceptual, definitional, and therapeutic issues regarding minor and subsyndromal depressions, murky enough in broader patient populations (
75–
78), remain largely uncharted territory in dying patients.
Given such limitations, along with the difficulties in conducting psychopathological research in dying patients, the empirical research literature on depression in dying patients is relatively small, limited largely to cancer and HIV patient populations. Still, the findings are generally comparable to those seen in broader medical illness groups. The following are important “take-home” points about depression in dying patients:
1. Depressive symptoms and syndromes are common in dying patients, although the range of estimated prevalence rates (approximately 15%–60%) likely reflects the heterogeneity of depressive conditions and their definitions as well as the patient populations (
79–
82).
2. As in primary care and other broad settings, self-reported depression inventories, including the single-item question “Are you depressed?”, have good operating characteristics as screens for diagnosable depressive disorders (
83–
85).
3. Evidence from patients with cerebro- or cardio-vascular disorders (
86,
87) or cancer (
88) suggest that depressive conditions in dying patients are multifactorial in origin, representing a combination of physiological effects of the disease process on brain functioning, premorbid diathesis toward depression, and current psychological and psychosocial factors.
4. Depression is associated with poorer will to live and greater desire for a hastened death (
89,
90). Together with extrapolations from data regarding functioning in a variety of medical populations (
91,
92), these data strongly suggest that depression is associated with excess functional morbidity and poorer quality of life in dying patients, making it an important target for palliative care.
5. Better treatment of chronic pain or other physical symptoms may result in improvement in concomitant depressive symptoms. Conversely, successful treatment of depression may reduce patient ratings of pain severity or pain-associated functional morbidity (
93).
6. Turning to depression treatments, to my knowledge there are no well-controlled trials of drugs or psychosocial treatments in terminally ill patients. Extrapolating from literature in chronically medically ill patients, depression in dying patients probably can respond to standard treatments (
91,
94), with resultant improvement in “quality of life” such as subjective distress and functional morbidity, although response rates may be lower than that seen in healthier populations. In the absence of further empirical data, choice of antidepressant drug should be guided by side-effect profile in the context of the patient's symptoms and comorbidities. Also, there may be a role for psychostimulants in targeting anergia, anhedonia, and abulia in patients who do not have a full major depressive syndrome, or in depressed patients with a very short life expectancy, in whom rapid symptomatic improvement is needed (
95–
99). Recently, the newer stimulant modafinil has been used (
100), although it is not yet clear whether its putatively better side-effect profile, compared with older agents such as methylphenidate (on the basis of its brain regional selectivity) results in better efficacy due to greater tolerability of higher doses.
Anxiety.
Although empirical prevalence data are relatively lacking, it is well recognized that anxiety is a common and distressing symptom in dying patients (
13,
14,
104). Anxiety may be comorbid with depression or delirium, or it may present alone as a primary condition or as secondary to medical illness or its treatments. Affective, ideational, or physiological manifestations of anxiety commonly accompany distressing physical symptoms such as pain, dyspnea, and vertigo. Anxiety in dying patients often occurs in a generalized anxiety pattern, although panic attacks or acute stress disorder-like symptoms (referable to the medical disease, its treatments, or to a feared mode of death) may be seen. Anxiety may contribute, along with depression, to a desire for a hastened death (
105). Geriatric psychiatrists will draw on their expertise in assessing anxiety in the context of such comorbidities.
Recommendations for treatment of anxiety are based largely on extrapolations from other populations, given that controlled trials in terminally ill patients are lacking. If rapid symptom control is needed because of acute distress or limited remaining life-span, benzodiazepines or (in the presence of delirium or significant dementia) antipsychotic medications are the treatments of choice, sometimes in combination with opioids or other sedatives (see below). Opioids may be particularly useful for anxiety in the context of dyspnea due to cardiac or pulmonary disease (
106,
107). If time available permits, antidepressant medications may be the primary treatment for generalized anxiety or panic-pattern symptoms (whether or not they are judged to be secondary to medical illness), or if the anxiety is comorbid with significant depressive symptoms. The role of other agents, such as buspirone, in treating anxiety in dying patients has not been well defined. Similarly, cognitive-behavioral or supportive therapy for anxiety may be useful in patients who have sufficient time, cognitive capacity, and motivation for treatment, although, again, empirical studies in terminal patients are few and are not well controlled (
108,
109).
Delirium.
Unsurprisingly, dying patients commonly develop “acute brain failure,” that is, the fluctuating global cognitive dysfunction of delirium related to primary CNS disease or to the CNS sequelae of systemic diseases or medications (
110,
111). Although cognitive impairment is the hallmark of the delirium syndrome, it is important to remember that other portions of the mental status examination may be prominently affected, for example, depression, anxiety, psychosis, or psychomotor disturbances. Often the manifestations of delirium considerably add to the patient's morbidity and to distress for the family. The standard approach to delirum evaluation, namely to identify and treat the underlying causes, may not always apply to dying patients. The causes may not be remediable, or may be iatrogenic in the service of palliation, for example, opioids or glucocorticoids. In many cases, work-up to determine the underlying cause(s) will be precluded by patient/family decisions to pursue a purely palliative approach. In any case, one should re-evaluate the benefit/risk profile of all CNS-active medications, balancing the helpful effects of such drugs with their potential contribution to the state of delirium. Symptomatic management often targets distressing components of delirium, such as agitation, psychosis, or affective lability. Although there may be some role for simple environmental interventions (i.e., decreasing unnecessary stimuli when possible), the mainstay of therapy for these symptoms remains antipsychotic medications. The second-generation antipsychotic medications have been used, but their advantage (presumably in terms of side-effect profile) over older antipsychotic drugs in terminal (or indeed any) delirium is not yet clear (
112). For intractable terminal delirium that produces severe or dangerous agitation or distress, other agents may be used alone or in combination with antipsychotics, including benzodiazepines, opioids, or anesthetic agents (see the section below on terminal sedation).
The geriatric psychiatrist is well versed in the assessment and management of delirium in the medically ill older patient. She or he also can serve a useful educational role for the family and sometimes for other medical providers, informing them about the cause of the distressing symptoms (e.g., that the emotional symptoms in delirium do not have a purely “psychological” origin and may not respond well to interpersonal interactions alone), and helping avoid the unnecessary and often counterproductive use of other psychotropic medications (e.g., hypnotics or antidepressants).
Role of supportive psychotherapy.
In addition to the psychiatric disorder-specific indications for focused psychotherapies, other forms of time-limited psychotherapy may be quite useful for selected patients or their families (
108,
109). Geriatric mental health specialists can draw on their knowledge of individual and family developmental issues as related to later life, as well as their specific psychotherapeutic skills. Particularly if contacts are begun earlier in the trajectory of an ultimately life-ending illness, individual psychotherapy may be used to facilitate life review, to help the patient set, prioritize, and achieve manageable goals in the time they have remaining, and, in some cases, to process longer-standing unresolved conflicts or dysfunctional patterns of thinking or interactions that affect the dying process. Some patients may come to view dying as an opportunity for psychological or spiritual growth, a possibility that physicians should suggest and support when possible (
113).
Family dynamics, of course, are a clinically crucial context within which the dying process takes place, and may be quite prominent in their effects on the treatment alliance and on needed decision-making. As with the individual, clinicians should assess the family to inform an approach that capitalizes on the family's strengths and shores up areas of weakness (
30). The mnemonic “L-I-F-E” has been suggested to organize approaches to brief family assessment, including Life-cycle stresses, Individual roles within the family, Family relational processes, and Ethnic factors (
114). Depending on the family's needs, interventions may range from education and community referral to deeper interventions, such as restoring effective problem-solving, improving patterns of communication, or improving attachment and caregiving bonds (
115). Family contacts also may be viewed as preventative: education and support may aid the grieving process even before the patient dies, optimally reducing the likelihood that bereavement will be complicated by depression or traumatic grief (
116), and increasing the likelihood or rapidity with which family members seek treatment for complicated bereavement.