AN EPIDEMIC WITHIN AN EPIDEMIC
Weight gain and metabolic dysregulation in patients taking second-generation antipsychotic medications constitute an epidemic within an epidemic. The proportion of all U.S. adults who are overweight or obese increased from 47% to 65% over the past two decades, after remaining stable over the previous two decades (
1), and the number of individuals with diabetes has more than doubled, from 5.8 to 14.7 million (
2). Although studies of schizophrenia patients before the use of second-generation antipsychotics suggest elevated rates of overweight and diabetes, substantial evidence from case reports, clinical trials, case registries, insurance databases, and government surveillance programs implicates some or all second-generation antipsychotics in causing or worsening weight gain, dyslipidemia, and diabetes (
3). The metabolic syndrome, a co-occurrence of interrelated risk factors including obesity, insulin resistance, dyslipidemia, hypertension, and a proinflammatory and prothrombotic state that appears to directly promote atherosclerotic cardiovascular disease, is emerging as the tardive dyskinesia of the second-generation antipsychotics.
GLUCOSE REGULATION, DIABETES, ADIPOSITY, AND DYSLIPIDEMIA
Insulin is secreted by beta cells of the pancreas and acts at receptors in muscle, liver, and fat to regulate glucose and lipid metabolism. After a meal, secreted insulin stimulates the uptake of glucose into skeletal muscle, inhibits the production of glucose by the liver (glycolysis), and inhibits the breakdown of lipids and release of free fatty acids from adipocytes (lipolysis). Type 1 diabetes, which accounts for less than 10% of diabetes cases, often begins in childhood and is usually the result of autoimmune destruction of the insulin-secreting pancreatic beta cells. Type 2 diabetes, which usually begins after age 45, is characterized by two pathological processes: inadequate insulin secretion and impaired insulin action at the insulin receptor, or insulin resistance. Early in the course of type 2 diabetes, insulin resistance, caused by genetic and/or environmental factors, evokes a compensatory increase in pancreatic insulin secretion so that glycemic control is maintained; insulin levels are elevated, but random and fasting plasma glucose levels remain normal. Insulin resistance and compensatory hyperinsulinemia are typically associated with elevated fasting triglyceride levels, low levels of high-density lipoprotein (HDL) cholesterol, and elevated levels of atherogenic low-density lipoprotein (LDL) cholesterol particles. Over a period of 7 to 10 years on average, increasing insulin resistance and/or deteriorating beta cell function leads to a state in which pancreatic compensatory capacity is overwhelmed (
4).
Insulin insufficiency is first evident as postprandial hyperglycemia (or an abnormal glucose tolerance test) due to impaired uptake of glucose into muscle. Later in the course of the disease, with progressive loss of insulin secretion, liver glucose production becomes dysregulated, resulting in fasting hyperglycemia. At this relatively advanced illness stage, an elevated fasting plasma glucose level allows detection of “prediabetes” or type 2 diabetes. Type 2 diabetes is diagnosed by measurement of fasting plasma glucose level using thresholds for diabetes (>125 mg/dl) and prediabetes (100–125 mg/dl) defined by the American Diabetes Association (
5).
With progressive beta cell failure, disinhibition of inhibition of lipolysis increases, further reducing control over free fatty acid release and worsening the characteristic dyslipidemia associated with diabetes. Physiological stress, such as intercurrent illness in the presence of marked impairment in insulin secretory functioning and insulin resistance, can result in severe hyperglycemia, which can acutely inhibit beta cell function, a state known as glucose toxicity. Under these circumstances, acute glycemic decompensation may result in diabetic coma and death due to extreme hyperglycemia with excessive fatty acid and ketone formation (diabetic ketoacidosis) or nonketotic hyperosmolar states.
Insulin resistance and type 2 diabetes occur most often in the context of overweight and obesity, particularly excess abdominal adiposity. Adiposity and fitness are each thought to contribute about 30% of the interindividual variance in insulin resistance, with genetic factors accounting for the remainder (
6). Thus, while excessive abdominal adiposity is significantly related to risk of insulin resistance and diabetes, type 2 diabetes can also occur in the absence of overweight or obesity.
METABOLIC SYNDROME
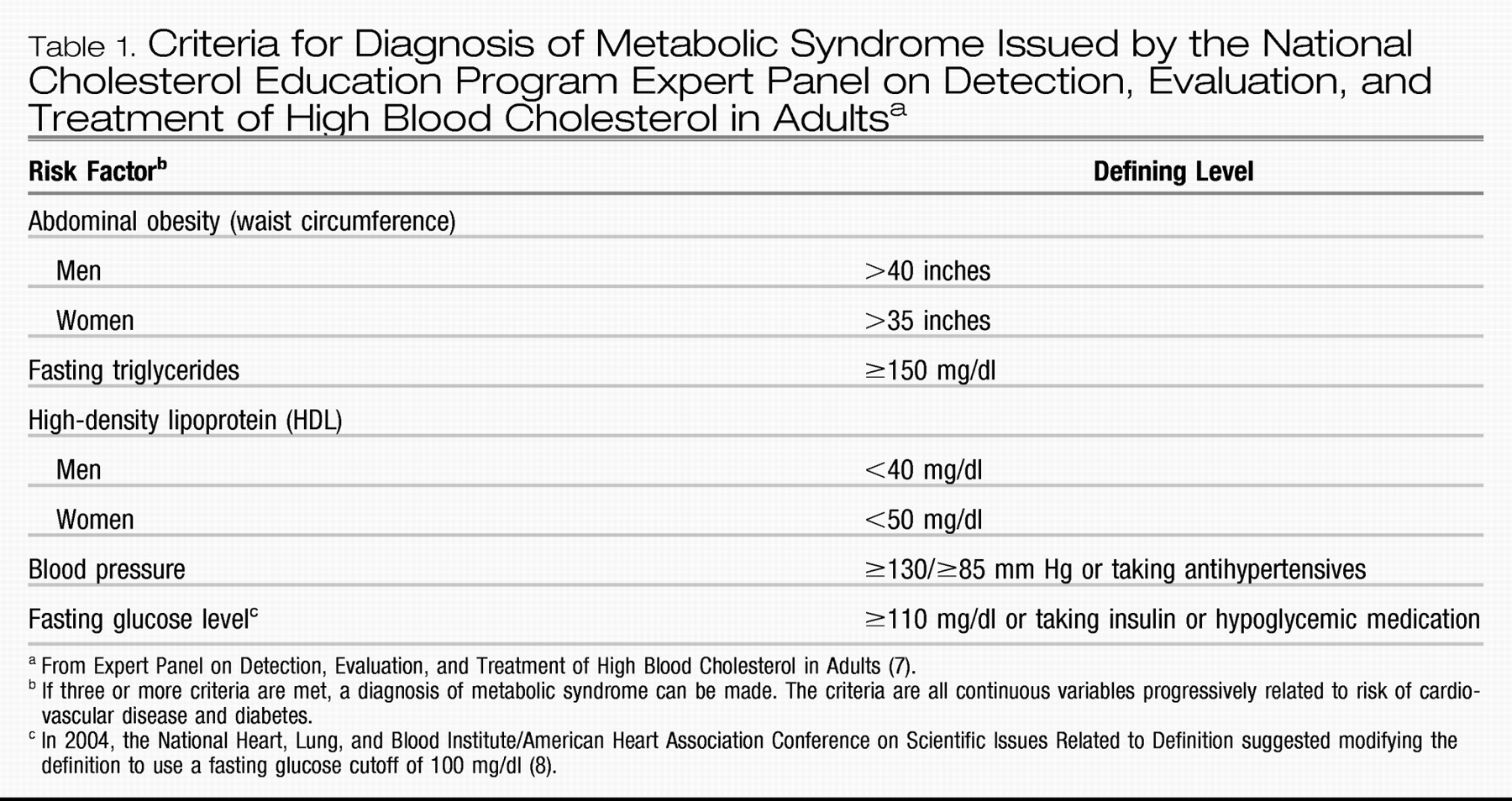
The interconnected pathophysiology of abdominal obesity, insulin resistance, hypertension, and disturbances in lipid metabolism can result in a co-occurrence of risk factors for cardiovascular disease and diabetes known as the metabolic syndrome (also called syndrome X). To increase awareness of these interrelated risk factors, the U.S. National Cholesterol Education Program Adult Treatment Panel III (
7) established criteria for the metabolic syndrome as a categorical public health construct (
Table 1). Cutoff values for individual elements of the metabolic syndrome are based in part on thresholds associated with an elevated risk of diabetes or cardiovascular disease.
In men, the metabolic syndrome is associated with a 25%–50% increase in risk of cardiovascular disease and mortality (
9). In one study of men 40–59 years of age, the probability of developing cardiovascular disease or type 2 diabetes over 20 years was 11.9% in those with no metabolic abnormalities, 31.2% in those with three abnormalities, and 40.8% in those with four or five abnormalities (
10). The metabolic syndrome increases the risk of cardiovascular disease to a greater extent in men than in women, but it is highly predictive of type 2 diabetes in both sexes (
8), and type 2 diabetes is itself highly predictive of cardiovascular disease. More than 20% of individuals with diabetes will develop coronary heart disease or have a recurrence of a coronary heart disease event within 10 years (
7).
Important potentially modifiable risk factors for coronary heart disease that are not subsumed in the definition of metabolic syndrome include elevated LDL cholesterol level, cigarette smoking, family history of premature coronary heart disease, and age ≥45 years for men and ≥55 years for women (
7). Analyses of the independent contribution of components of the metabolic syndrome added to these risk factors suggest that these components are not all equally associated with risk of coronary heart disease; most of the risk associated with the metabolic syndrome is captured by age, blood pressure, cholesterol, diabetes, and HDL cholesterol (
8). Because of the differential risk across components of the metabolic syndrome (for example, waist circumference may add little risk compared with low HDL, while HDL alone, without other elements of the metabolic syndrome, is a significant independent risk factor), some have argued that the “diagnosis” of metabolic syndrome is an artificial construct that is less informative than the sum of its parts (
6,
11). Others contend that the concept, while perhaps failing to “carve nature at its joints,” has succeeded in calling attention to an important problem and productively driven research and clinical collaboration between cardiologists, endocrinologists, and general practitioners (
12). Controversies concerning the validity of the metabolic syndrome as a categorical diagnosis bear a striking resemblance to debates about the validity of psychiatric diagnoses based on operational criteria that define disorders from symptom and severity thresholds that are at least partly arbitrary.
EPIDEMIOLOGY OF THE METABOLIC SYNDROME IN SCHIZOPHRENIA
The largest sample of persons with schizophrenia for which the rate of metabolic syndrome has been reported is that of the National Institute of Mental Health-sponsored Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) (
13). The prevalence of metabolic syndrome (using modified criteria; see
Table 1, note c) was 42.7% of 689 assessable patients. Mean BMI was 29.7 (SD = 7.0) (a BMI of 25–29.9 is considered overweight, and ≥30 is considered obese). Among fasting subjects (N = 342), 44.4% met criteria for the metabolic syndrome; the proportion meeting individual items were waist circumference, 39%; BP, 45.9%; triglycerides, 58.3%; HDL, 55.1%; glucose >100, 26.5%. Adjusted odds ratios of metabolic syndrome status for CATIE subjects relative to matched individuals in the National Health and Nutrition Examination Survey (NHANES) III were twice as high for men in the CATIE sample (odds ratio = 2.297, p < 0.0001) and three times as high for women (odds ratio = 3.186, p < 0.0001). These data clearly indicate that the risk of metabolic syndrome in schizophrenia patients in nearly all age groups is 2–3 times that of the general population (
13; see also
14).
ANTIPSYCHOTIC DRUGS AND METABOLIC SYNDROME: PATHOPHYSIOLOGY
Because an elevated risk of diabetes was noted in patients with schizophrenia before the introduction of chlorpromazine, the risk may be related to disease or life style factors. First-generation antipsychotics themselves, particularly low-potency phenothiazines, are associated with significant weight gain and increases in plasma lipids (
15). Recent awareness of metabolic side effects of antipsychotics was triggered by reports of dramatic weight gain, diabetes, deaths from ketoacidosis, and atherogenic lipid profiles following the introduction of clozapine in the United States in 1990. These adverse outcomes have been most associated with clozapine and olanzapine (
3,
15).
In most cases, weight gain and abdominal adiposity may be central to the pathophysiology of metabolic syndrome induced by second-generation antipsychotics. Increasing visceral adiposity (which can be assessed by measuring waist circumference or estimated by BMI) is directly associated with insulin resistance, dyslipidemia, and risk of diabetes.
The second-generation antipsychotics vary in their propensity to induce weight gain; clozapine and olanzapine produce the most weight gain, quetiapine and risperidone produce intermediate weight gain, and ziprasidone and aripiprazole produce the least weight gain (
16,
17). Available evidence suggests that differences in weight gain associated with these agents reflect their order of risk for insulin resistance, glucoregulatory dysfunction, and dyslipidemia (
15,
17).
The mechanisms by which antipsychotic medications produce weight gain may include stimulating appetite, reducing physical activity, and directly impairing metabolic regulation. The daily balance between calories consumed and expended determines an individual's weight, and even small imbalances can cause significant changes in weight. On average, a 3% increase in daily caloric intake (the equivalent of one soft drink and a bag of potato chips) without an increase in energy expenditure results in a 10-lb. weight gain over a 1-year period (
18). Many drugs marketed to induce weight loss suppress appetite and hunger by enhancing the action of monoamine neurotransmitters (serotonin, norepinephrine, and histamine) in the CNS (
19). Conversely, many second-generation antipsychotics inhibit or reduce the activity of these same neurotransmitters and thus may increase appetite.
A recent mouse study reported weight gain associated with olanzapine, quetiapine, risperidone, and ziprasidone but increased food intake only with olanzapine and quetiapine (
20). It has been difficult to translate findings from animal models of feeding regulation to humans, and further research is needed to quantify the role of medicationinduced increases in food intake in weight gain associated with second-generation antipsychotics.
Not all antipsychotic-induced metabolic disturbances result from increased adiposity. Some patients taking second-generation antipsychotics experience new-onset diabetes without changes in weight, and experimental studies demonstrate medication-associated insulin resistance independent of adiposity (
21). Metabolic disturbances related to second-generation antipsychotics may result from a direct alteration of insulin sensitivity and/or insulin secretion. Antipsychotic affinity at both histamine and muscarinic acetylcholine receptors correlates with weight gain and metabolic liability (
22), and impaired parasympathetic regulation of beta cell activity may contribute to metabolic risk (
23). Insulin sensitivity may also be reduced as a result of alterations in gene products in the insulin-signaling pathway and/or elevated levels of circulating factors that alter the insulin signaling. For example, there is evidence that certain antipsychotic agents may directly impair glucose transporter function. Glucose transporters are regulated by insulin and actively transfer glucose into peripheral tissues (e.g., liver, muscle, and fat). Direct attenuation of glucose transporter function by antipsychotic agents would result in elevations in circulating glucose and a compensatory hypersecretion of insulin, which over time may further reduce insulin sensitivity, triggering the cascade of events leading to metabolic syndrome and type 2 diabetes (
24).
SECOND-GENERATION ANTIPSYCHOTICS: DIFFERENTIAL RISK OR DRUG CLASS EFFECT?
The pathophysiology of antipsychotic-induced insulin resistance and metabolic disturbance is not fully understood. Despite expert opinion that the risk of diabetes and metabolic syndrome for the various second-generation antipsychotics is proportional to their association with weight gain (
3,
17), reports of incident diabetes in the absence of weight gain and a paucity of epidemiological data and randomized clinical trials to directly address risk in newer second-generation antipsychotics leave unanswered the question of whether second-generation antipsychotics that cause little or no weight gain also carry little or no risk of diabetes or metabolic syndrome. Concluding that available data do not allow the ranking of diabetes risk across second-generation antipsychotics, effective June 2004 the Federal Drug Administration (FDA) has required a warning concerning the risk of treatment-emergent hyperglycemia for all marketed drugs in this class (
25). In contrast to the FDA position, the American Diabetes Association and American Psychiatric Association (ADA/APA) Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes, on the basis of differential weight gain in clinical trials and data from cohort studies, ranks clozapine and olanzapine as most associated, risperidone and quetiapine as less clearly associated, and ziprasidone and aripiprazole as probably not associated with an increased risk of diabetes. Nonetheless, because patients with schizophrenia represent a high-risk group generally, the ADA/APA Consensus Development Conference recommends metabolic monitoring for all patients taking second-generation antipsychotics (
17), a recommendation also endorsed by the FDA (
26).
MANAGEMENT OF METABOLIC SYNDROME: MEDICAL PERSPECTIVE
From the general medical perspective, cardiovascular disease is the primary clinical outcome of the metabolic syndrome. Hence, assessment and treatment of the metabolic syndrome are embedded in overall management of the risk of cardiovascular disease. Because elevated LDL cholesterol is associated with a heightened risk of cardiovascular disease and interventions that lower LDL reduce this risk, lowering LDL cholesterol is the primary goal of therapy. Individualized patient assessment stratifies patients into categories based on risk factors for cardiovascular disease (age, cholesterol, blood pressure, and cigarette smoking), and patients in progressively higher risk categories are treated to progressively lower LDL cholesterol target goals (
7). The Adult Treatment Panel III treatment guidelines (
7) recommend therapeutic life style changes, including reduced intake of saturated fats and cholesterol, increased fiber intake, weight reduction, and increased physical activity as the first-line therapeutic approach to the risk of cardiovascular disease. LDL-lowering drugs, including HMG-CoA reductase inhibitors (statins), bile acid sequestrants, nicotinic acid, and fibric acids, are prescribed as needed to achieve target LDL levels.
The metabolic syndrome increases the risk of cardiovascular disease at any given level of LDL and is considered a secondary target of risk-reduction therapy after lowering LDL cholesterol (
7). The Adult Treatment Panel III guidelines (
7) identify obesity as the primary target of treatment of the metabolic syndrome and weight loss and increased physical activity as the first-line treatment approaches. Weight loss lowers LDL cholesterol and triglycerides, increases HDL cholesterol, lowers blood pressure, and reduces insulin resistance. While the benefits of weight reduction are unequivocal, attaining them through behavior change is often extremely difficult (
8).
In addition to overweight and obesity, each element of the metabolic syndrome can be considered an independent treatment target to reduce the risk of cardiovascular disease (
27). Metformin reduces insulin resistance, reduces new-onset coronary heart disease in obese patients with diabetes, and prevents or delays type 2 diabetes in patients with impaired glucose tolerance (
8,
28). Insulin sensitizers of the thiazolidinedione class also prevent or delay type 2 diabetes in at-risk patients (
27). Neither has been tested extensively in patients with impaired glucose tolerance induced by second-generation antipsychotics. Hypertensive patients who are taking second-generation antipsychotics and meet criteria for the metabolic syndrome should be treated with therapeutic life style changes and medications in accordance with hypertension guidelines (
29). Finally, low-dose aspirin may be indicated to mitigate the prothrombotic state in patients with metabolic syndrome at elevated risk of coronary heart disease.
MANAGEMENT OF METABOLIC SYNDROME: PSYCHIATRIC PERSPECTIVE
From the psychiatric perspective, the risk of metabolic syndrome associated with second-generation antipsychotics imposes new standards for patient education, informed consent, and risk-benefit analysis in the selection of pharmacotherapy. In addition, these risks create new monitoring requirements and a responsibility to ensure appropriate provision of general medical care for patients. The ADA/APA Consensus Development Conference recommends a baseline assessment of personal and family history of risk factors for cardiovascular disease (obesity, diabetes, hypertension, cardiovascular disease), weight and height (for BMI calculation), waist circumference, blood pressure, fasting plasma glucose level, and fasting lipid profile before initiating treatment with a second-generation antipsychotic and at periodic intervals during treatment (
17). These guidelines suggest consideration of switching to a second-generation antipsychotic with less weight gain liability if a patient gains more than 5% of his or her initial weight or develops worsening dyslipidemia or hyperglycemia. The guidelines recommend specialist referral for patients who develop diabetes or hypertension.
Data from the recent CATIE trial, however, point to what may be a relatively common clinical dilemma. The second-generation antipsychotics with the greatest effectiveness (at least at the doses tested in CATIE), were also associated with the greatest metabolic side effects (
30). Consequently, for some patients the risk of illness exacerbation due to loss of efficacy may militate against attempting to switch to newer second-generation antipsychotics with less weight gain liability. Given the unpredictable interindividual response to these agents, the reality that it can take several years to establish an adequate medication regimen, and the cost of relapse, some have argued that efficacy should be the prime determinant in the choice of an antipsychotic medication (
31). This approach advocates consideration of switching only after other options have been exhausted. With the exception of the superiority of clozapine in treatment-resistant schizophrenia, evidence from head-to-head comparisons of second-generation antipsychotics provides little guidance on comparative efficacy of these agents relative to each other (
32). Thus, the CATIE findings suggesting a trade-off between efficacy and side effects may be spurious. In view of the paucity of independent evidence to address this question, a shared decision-making model that accounts for the individual patient's values, risk tolerance, and preferences should guide clinical practice.
A variety of pharmacological agents have been studied in efforts to reverse weight gain induced by second-generation antipsychotics. Typically, only modest weight reductions are reported, and current evidence is insufficient to support any particular pharmacological approach (
33–
35). A pilot study found surgical treatment of morbid obesity in five schizophrenia patients safe and effective (
36). Very few prevention-oriented studies have been conducted. A small trial of amantadine in patients who were already being treated with olanzapine appeared to attenuate further weight gain (
37). In contrast, neither nizatidine (
38) nor metformin (
39) prevented weight gain in patients taking olanzapine. Prevention research targeting first-episode patients before any significant weight gain occurs is clearly needed. In the absence of effective pharmacological approaches to weight gain, life style modification remains the promising treatment option.
Obesity guidelines for nonpsychiatric populations define a number of principles that are applicable to patients with weight gain associated with second-generation antipsychotics, including the following: 1) The initial goal of weight loss therapy should be to reduce body weight by approximately 10% from baseline; extreme diets are seldom effective in producing long-term weight reduction. 2) Weight loss targets should be about 1–2 lbs. per week for a period of 6 months, with the subsequent strategy based on the amount of weight lost. 3) Weight loss and weight maintenance therapy should employ the combination of lowcalorie diets, increased physical activity, and behavior therapy. 4) Weight loss drugs should never be used without concomitant life style modifications and continual assessment of drug therapy for efficacy and safety. If the drug is efficacious and there are no serious adverse effects, it can be continued. If not, it should be discontinued. 5) After successful weight loss, the likelihood of weight loss maintenance is enhanced by a program consisting of dietary therapy, physical activity, and behavior therapy that should be continued indefinitely. 6) Weight loss and weight maintenance therapies that provide a greater frequency of contacts between the patient and the practitioner and are provided over the long term should be used whenever possible (
40).
Behavioral approaches to achieving weight loss in patients with schizophrenia have been used with variable success, but most studies are anecdotal and/or methodologically limited (
41). Pilot programs involving direct management of diet for patients in supervised-living settings (
42) and direct participation in exercise group activities (
43) show promise in slowing or reversing weight gain for some patients. Outpatient group behavior therapies have used educational, motivational, and pragmatic techniques (
44). Further development and testing of theory-based behavior change interventions tailored to the special needs of individuals with serious mental illness are warranted.
PSYCHIATRIC AND GENERAL MEDICAL TREATMENT INTEGRATION
While management of the metabolic syndrome in patients taking second-generation antipsychotics often requires collaboration between a psychiatrist and a general medical physician, the integration of general medical services into health settings where patients with serious mental illness are typically seen is underdeveloped. As a consequence, patients with serious mental illness may not receive general medical care or may be at risk of receiving poorer quality care (
45). Collaborative care service models (generally built around a designated nurse care coordinator) have been piloted (
46) but are not widely implemented. A recent consensus meeting concluded that because mental health care providers have the most direct contact with schizophrenia patients, they should have the capacity to conduct basic health screening, including assessment and monitoring of each of the elements of the metabolic syndrome (
47). At a minimum, based on clinician experience and local circumstances, treatment plans for patients on second-generation antipsychotics should indicate explicitly who is responsible for ongoing monitoring of metabolic risk.
SUMMARY AND RECOMMENDATIONS
The patient described at the beginning of this article meets four of the five criteria for the metabolic syndrome: waist circumference, total triglycerides, HDL cholesterol, and blood pressure. The presence of low HDL cholesterol and a family history of premature death from coronary heart disease places Mr. P at an intermediate risk of coronary heart disease over the next 10 years. In addition, Mr. P has prediabetes, defined as a fasting serum glucose >99 mg/dl but <126 mg/dl. Given his obesity, positive family history of diabetes, and low HDL level, Mr. P has an elevated risk of developing type 2 diabetes over the next several years. Evaluation by an internist is indicated to further assess for the presence of coronary heart disease. Because the initial assessment yielded no current evidence of coronary heart disease, the major goal of medical intervention is primary prevention. The Adult Treatment Panel III guidelines (
7) recommend therapeutic life style changes to 1) reduce intake of saturated fat and cholesterol, 2) increase physical activity, and 3) support weight control.
These measures were implemented, but because they did not lower LDL levels to the target range (130 mg/dl for this patient) after 12 weeks, an LDL-lowering statin was prescribed. Also, given the patient's difficulty in sustaining life style changes, metformin was prescribed to reduce the probability of prediabetes progressing to full-blown type 2 diabetes. Mr. P's treatment plan calls for his psychiatrist to monitor the parameters of metabolic syndrome quarterly and to inform the patient's internist of changes between biannual general medical visits. Mr. P was encouraged to purchase a scale and record his weight weekly. With weight monitoring now a routine part of his appointment with his psychiatrist, diet and weight control efforts became an active topic of discussion during psychiatric visits.
Mr. P's social isolation was also a major focus of treatment, and with encouragement, he agreed to attend a weekly peer support group. Although some peer support and rehabilitation-oriented programs integrate behavioral approaches to exercise and physical health into their offerings, in this patient's case, it was a desire for a better social life that catalyzed behavior change. Greater contact with peers rekindled Mr. P's interest in the opposite sex. He was too shy and too embarrassed about his weight to engage in dating, so he enrolled in an Internet-based dating service. With the semianonymity of online dating, he established a relationship with a single woman who also suffered from disability. After many months, as the issue of when and how to meet face-to-face became salient, Mr. P arrived at a solution: they would delay the meeting for 6 months, which would give him time to lose some weight. He was now amenable to a consultation with a nutritionist to learn skills required to execute this plan. A significant barrier to regular exercise was removed when his sister helped him join a health club. Where medical and educational efforts alone had fallen short, life events intervened to solidify the patient's intention and motivation to become healthier in order to achieve a meaningful personal goal.