I have been asked to consult on a patient who suffers from delirium. What are the pertinent risk factors and are there any good treatment algorithms?
Delirium is a challenging neuropsychiatric problem affecting medically ill patients. It is also the most common psychiatric syndrome found in the general hospital setting. Its prevalence surpasses that of the most commonly known and identified psychiatric syndromes and varies depending on the medical setting (
1). By definition, delirium is an acute or subacute organic mental syndrome characterized by disturbance of consciousness, global cognitive impairment, disorientation, the development of perceptual disturbance, attention deficits, disordered sleep-wake cycle, fluctuation in presentation (e.g., waxing and waning), and changes in psychomotor activity (depending on the type of delirium) (
1).
Seldom are we able to identify a single clear cause for the development of delirium in any one patient. Therefore, the syndrome of delirium is better understood as having a multifactorial etiology. These multiple etiological entities may give rise to transient disruption of normal neuronal activity, which in turn cause the various manifestations of delirium. Details of the pathogenesis of delirium have been discussed extensively elsewhere (
2).
Of the many risk factors identified as causative of delirium (
1), the following are the most common:
Age. Studies have suggested that age is an independent predictor of a transition to delirium (
3,
4). The increased incidence of delirium in older patients may be associated with a decrease in the volume of acetylcholine-producing cells occurring during the normal aging process (
5). Data suggest that the probability of transitioning to delirium increases dramatically (by 2%) for each year of life after age 65 (
6). Others have demonstrated that older patients have a higher incidence of development of postoperative delirium, even after relatively simple outpatient surgery (
7).
Baseline cognitive level of functioning. Studies in both the acute medical ward and surgical units suggest that the presence of baseline cognitive decline or dementia increases the occurrence of delirium (
3,
4,
8–
10). A study of elderly subjects undergoing joint replacement demonstrated that the presence of dementia significantly increased the occurrence of delirium (31.8% in the population without dementia versus 100% of subjects with dementia) (
11). Yet, even in individuals without dementia, the presence of subtle preoperative attention deficits was closely associated with a four to five times increase in the development of postoperative delirium (
12).
Gender. Studies suggest that most patients who develop delirium are male (63% male versus 37% female) (
3,
13).
Sensory impairment. Of the various forms of sensory impairment, only visual impairment has been shown to contribute to delirium (
4). In fact, studies have shown that visual impairment can increase the risk of delirium 3.5-fold (
14).
Exogenous substances. Many substances have been associated with a greater deliriogenic potential, including benzodiazepines (
3,
6,
15,
16), corticosteroids (
16,
17), and opioids (
16–
18). For example, studies have shown that the probability of transitioning to delirium increased with the dose of lorazepam administered in the previous 24 hours. This incremental risk was large at low doses and plateaued at around 20 mg/day (
6,
19). Yet the most direct evidence indicates that a substance's anticholinergic potential is related to delirium (
20,
21). In fact, some have demonstrated that the threshold for delirium occurs when the anticholinergic serum level reaches 0.80 ng/ml (
21).
Endogenous anticholinergic substances. Studies have demonstrated that detectable serum anticholinergic activity (SAA) have been found in delirious patients who were not exposed to pharmacologic agents with known anticholinergic activity. These findings suggest that endogenous anticholinergic substances may exist during acute illness and may be implicated in the etiology of delirium (
22–
24).
Immobility and physical restraints. The use of restraints, including endotracheal tubes (ventilator), soft and leather restraints, intravenous lines, bladder catheters, and intermittent pneumatic leg compression devices, casts, and traction devices all have been associated with an increased incidence of delirium (
1,
4,
25).
Sleep deprivation. Studies have demonstrated that sleep deprivation may lead to the development of both psychosis (
26) and delirium (
27–
30). Studies have found that the average amount of sleep in patients in an intensive care unit (ICU) is limited to 1 hour and 51 minutes per 24-hour period (
31). Many factors may affect sleep in the ICU, including frequent therapeutic interventions, the nature of diagnostic procedures, pain, fear, and the noisy environment (
1). Mounting data suggest that a cumulative sleep debt may not just be a cause of but may aggravate or perpetuate delirium (
32–
35). Among other factors, findings suggest that the dyssynchronization of the melatonin secretion rhythm commonly found among critical care patients (possibly mediated or exacerbated by the use of sedative agents) may contribute to the development of delirium (
36–
38).
Oversedation. Similarly, oversedation has been found to be an independent predictor of prolonged mechanical ventilation (
39). Sedative agents (mostly GABA-ergic) and opioids may contribute to the development of delirium by one of five mechanisms (
1): 1) interfering with physiological sleep patterns; 2) interfering with central cholinergic function muscarinic transmission at the level of the basal forebrain and hippocampus (i.e., causing a centrally mediated acetylcholine-deficient state); 3) increasing compensatory up-regulation of
N-methyl-
d-aspartate and kainite receptors and Ca
2+ channels; 4) disrupting the circadian rhythm of melatonin release; and 5) disrupting the thalamic gating function.
Psychiatric Disorders. Certain psychiatric diagnoses, including a history of alcohol and other substance abuse, as well as depression, schizophrenia and bipolar disorder have been associated with a higher incidence of delirium (
3,
40,
41).
Pain. Data suggests that both pain and medications used for the treatment of pain have been associated with the development of delirium. Studies have demonstrated that the presence of postoperative pain is an independent predictor of delirium after surgery (
42). On the other hand, the use of opioid agents has been implicated in the development of delirium (
43–
45). For example, opioids are blamed for nearly 60% of the cases of delirium in patients with advanced cancer (
46).
Severity of underlying medical illness/comorbidities. Evidence shows that the probability of transitioning to delirium increases dramatically for each additional point in the Acute Physiology and Chronic Health Evaluation (APACHE II) severity of illness score (
6). Others have also found that high medical comorbidity is an independent risk factor for the development of delirium (
3,
4).
It is impossible to cover all possible risk factors and mechanisms of delirium production in this commentary, but these have been thoroughly discussed in two recent review papers (
1,
2).
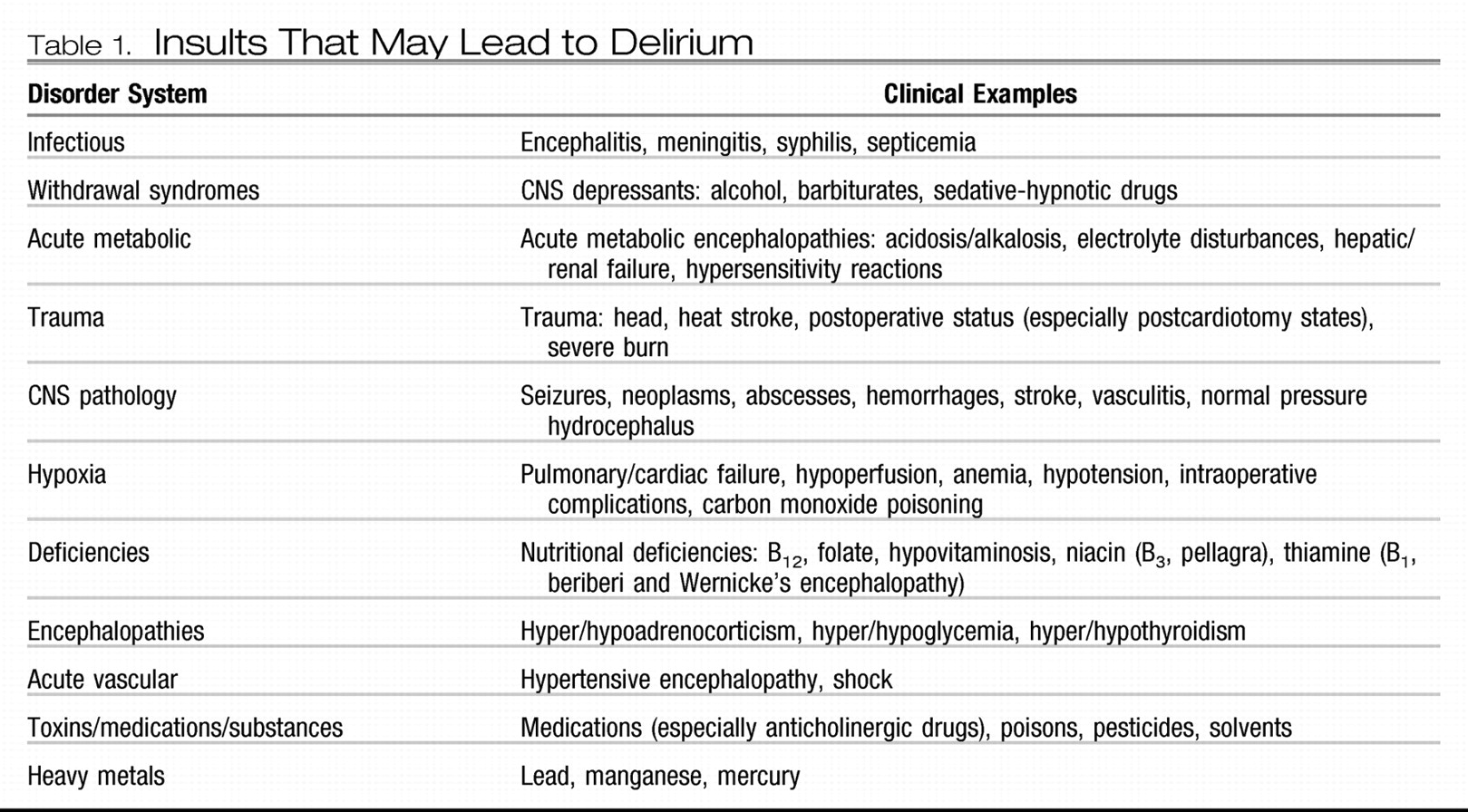
Table 1 also has a useful mnemonic for insults that may lead to delirium: “I watch death.”
Another important aspect to consider is the differential diagnosis. The symptoms of delirium vary from patient to patient and sometimes within a single patient over time. Some have suggested that there are motor variations (i.e., hyperactive, hypoactive, and mixed states) regarding their presentation (
47). But what a clinician needs to keep in mind is that symptoms of delirium may present like mania, psychosis, catatonia, and even depression. In fact, data suggests that nearly 40% of the times when psychosomatic medicine consultants are asked to assist in the management of depression of hospitalized medically ill patients, these subjects were, in fact, experiencing hypoactive delirium and not major depression (
48,
49). Substance intoxication/abuse (particularly CNS stimulant agent intoxication [e.g., cocaine and amphetamines]) and withdrawal states (particularly from CNS depressant agents [e.g., alcohol, benzodiazepines, and barbiturates]) may all lead to various forms of delirium (usually the hyperactive type). Conversely, acute intoxication with a CNS depressant or withdrawal from a CNS stimulant may lead to hypoactive forms of delirium.
Understanding delirium is important because of its high prevalence and the increased morbidity and mortality rates associated with its development. The incidence of delirium in the ICU has been reported to be as high as 81.3% (
50). Several studies have found that patients who developed delirium fared much worse than their counterparts without delirium when controlling for all other factors. One study (
51) showed that the mortality rate was higher among patients with delirium (as high as 8% compared with 1% in patients without delirium). In another study, patients in the ICU who developed delirium had higher 6-month mortality rates (34% versus 15%, p=0.03) (
52). Similarly, another study showed that the 90-day mortality was as high as 11% among patients with delirium, compared with only 3% among elderly patients without delirium (
53). Not only is delirium associated with increased mortality, but the rate of morbidity is also increased. Multiple studies have demonstrated that patients with delirium have prolonged hospital stays (i.e., average 5–10 days longer), compared with patients with the same medical problem who do not develop delirium as a complication (
50–
52,
54).
Finally, the development of delirium has been associated with significant increases in the cost of delivering care. A study of cardiac surgery demonstrated that the calculated additional cost caused by the development of postoperative delirium after cardiac surgery was $6,150 per patient (
55). Similarly, a subsequent study of the same population demonstrated that the development of postoperative delirium nearly doubled the cost of care in cardiac valve replacement (i.e., $6,763 in patients who did not develop delirium versus $12,965 in patients who developed delirium). A study of a step-down critical care unit showed that even though only 14% of patients developed delirium, they represented 22% of ALL hospital days during the index period (
54). The same study showed that patients with delirium remained hospitalized an average of 9.2 days longer than their counterparts without delirium at an average cost of $28,000 per patient. A study of patients in the ICU with delirium showed that health care costs were 31% higher than for patients with similar medical problems but without delirium (i.e., $41,836 versus $27,106 per patient) (
56). In addition, in a study of hospitalized elderly patients, data show that 1) delirium is common, 2) patients with delirium had significantly higher unadjusted health care costs and survived fewer days, 3) the average costs per day among patients with delirium were 2.5-fold greater than those for patients without delirium; and 4) the total cost estimates attributable to delirium ranged from $16,303 to $64,421 per patient (
57).
As can be surmised from the above facts, it is imperative to treat delirium early, but it is more important to prevent it. There are several excellent studies suggesting potential interventions for the prevention of delirium. The best nonpharmacological method is the multicomponent intervention method described below (
58). With this intervention strategy a 40% reduction in the odds of developing delirium has been seen. Other nonpharmacological prevention strategies, primarily consisting of the prophylactic use of neuropsychiatric consultation in “at-risk patients” have also demonstrated a lower incidence of postoperative delirium and a reduction in associated complications (e.g., decubitus ulcers, urinary tract infections, nutritional complications, sleeping problems, and falls) (
59,
60). There have also been pharmacological interventions in small, but promising studies that have demonstrated significant reductions in the development of delirium. Several studies have demonstrated that antipsychotics, given preoperatively, may reduce the incidence of postoperative delirium (
61–
64). Some studies have suggested that the long-term use of acetylcholinesterase inhibitors in at-risk populations may lower the incidence of postoperative delirium (
65,
66). However, studies using short-term preoperative treatment failed to demonstrate any benefit (
67,
68). A recently published article demonstrated a dramatic reduction in the incidence of postoperative delirium (3% versus 50%) in cardiac patients with the use of the novel anesthetic dexmedetomidine and avoidance of the use of more conventional GABA-ergic agents (
69).
There are few conclusive studies demonstrating efficacy in treatment. Many studies using pharmacological agents have shown either conflicting findings, or the study population or data size is inadequate to allow for generalizability of the results. Thus, what follows is a basic algorithm for the prevention and management of delirium. Steps associated with robust evidence-based data are identified with an asterisk (*). The others have been developed from clinical practice or empirical data based on theoretical models. A more thorough discussion of these, along with their theoretical rationale, is beyond the scope of this commentary and can be found elsewhere (
1,
2,
70–
72).
I.
Timely diagnosis
A.
Be vigilant for the possibility of delirium; remember that many symptoms may be confused with other psychiatric syndromes, particularly depression in cases of hypoactive delirium and agitation in cases of substance intoxication and withdrawal.
B.
Obtain information on the patient's baseline level of cognitive functioning from all available accessory sources (e.g., spouse, family, or nursing staff).
C.
Screen for the development of delirium in high-risk groups. Use objective delirium rating scales if possible [e.g., Delirium Rating Scale-98 (
73) or confusion assessment method (
74)].
II.
Identify and treat underlying medical problems that may be causing or contributing to delirium in your particular patient (
Table 1). The ultimate treatment of choice is the timely discovery and correction of the underlying medical causes of delirium. That is, aggressively treat infectious processes and electrolyte imbalances, correct vital signs and end-organ functioning, restore a more physiological sleep-wake cycle, minimize fear, anxiety, and pain, and manage extrinsic/environmental factors, such as lighting and noise control.*
III.
Institute non-pharmacological treatment strategies* (based on Inouye's multicomponent intervention method for prevention of delirium in elderly patients) (
58).
A.
Correct malnutrition, dehydration, and electrolyte abnormalities as quickly and safely as possible.
B.
Remove immobilizing lines and devices (i.e., intravenous lines, chest tubes, bladder catheters and physical restraints) as early as safely possible.
C.
Correct any sensory deficits the patient may have (i.e., eyeglasses or hearing aids).
D.
Promote as normal a circadian sleep pattern as possible. It is better if this can be achieved by environmental manipulations, such as light control (i.e., lights on and curtains drawn during the day and lights off at night) and noise control (i.e., provide ear plugs, turn off TVs, and minimize night staff chatter) rather than by the use of medications.
E.
Provide adequate intellectual and environmental stimulation as early as possible (e.g., orient the patient to date, time, and circumstance regularly, provide a newspaper, or set the TV to a news broadcast rather than to reruns).
F.
Minimize environmental isolation.
IV.
Consider pharmacological treatment strategies.
A.
Conduct an inventory of all pharmacological agents being administered to the patient. Any medication or agent known to cause delirium or to have high anticholinergic potential should be discontinued, if possible, or a suitable alternative should be instituted.*
B.
Avoid using GABA-ergic agents to control agitation, if possible.* Exception: In patients undergoing CNS depressant withdrawal (i.e., alcohol, benzodiazepines, or barbiturates) or when more appropriate agents have failed and sedation is needed to prevent patient's harming himself or herself.
C.
Adequately assess and treat pain because uncontrolled pain has been found to be a contributor to the development and exacerbation of delirium.*
D.
Avoid the use of opioids for behavioral control of agitation (i.e., use only for pain management), because opioid use has been associated with delirium.*
E.
For the pharmacological target management of delirium (all types) consider using the following.
1.
Acetylcholinesterase inhibitor (e.g., rivastigmine, donepezil, physostigmine, or rivastigmine) for correction of central anticholinergic syndrome.
2.
Serotonin antagonist (e.g., ondansetron), to control toxic elevations of serotonin usually associated with hypoactive delirium, although some studies have suggested use of a serotonin antagonist may be indicated in all types of delirium.
3.
Opioid agent rotation, such as switching from morphine and meperidine to fentanyl or hydromorphone.
4.
Melatonin or melatonin agonists (e.g., ramelteon) to promote a more natural sleep.
5.
Dopamine antagonists to manage the theorized abnormally elevated levels of dopamine and provide restoration of putative hippocampal functions (e.g., short-term memory) and reversal of other regional brain disturbances (e.g., agitation, psychosis, or primitive reflexes), as well as to protect neurons against hypoxic stress and injury (
2,
75). The dose of dopamine antagonist used may depend on the type of delirium being treated (
1,
76–
79).
6.
α
2 Adrenergic agonists (e.g., dexmedetomidine or clonidine) for protection against the acute release of norepinephrine due to hypoxia or ischemia, which leads to further neuronal injury and the development or worsening of delirium. The data to date are more robust for delirium prevention (
69,
80),* although data are emerging for treatment, especially delirium associated with massive norepinephrine discharges (i.e., alcohol withdrawal).
7.
N-methyl-d-aspartate receptor blocking agents to minimize glutamine-induced neuronal injury (e.g., amantadine or memantine).
F.
For hyperactive delirium:
1.
Use low to moderate doses of haloperidol (e.g., <20 mg/24 hour), if the patient's cardiac condition allows it and there are no significant electrolyte abnormalities.*
a.
Before using haloperidol, obtain a 12-lead electrocardiogram and measure the corrected Q-T interval (QTc) and electrolyte levels. Correct potassium and magnesium levels, if needed.
b.
If possible, avoid the use of other medications known to increase QTc and/or inhibitors of CYP3A4.
c.
Discontinue use of haloperidol if QTc increases to >25% of baseline or >500msec.
2.
When the use of haloperidol is contraindicated or not desirable (i.e., prolonged QTc or history of severe extrapyramidal symptoms), atypical antipsychotics should be considered (
1,
81):
a.
Evidence is better for risperidone and quetiapine.*
b.
Limited data are available for olanzapine, aripiprazole, and perospirone.
c.
Avoid clozapine and ziprasidone.
Note: antipsychotics should be used with caution and only short term for the management of delirium or agitation in patients suffering from dementia. Data suggest a twofold increase in mortality for patients with dementia after long-term treatment with antipsychotic agents (
82).
G.
For hypoactive delirium:
1.
Evidence suggests that dopamine antagonists may still have a place, given the excess dopamine theory.
a.
If haloperidol is used, recommended doses are in the very low range (i.e., 0.25–1 mg/24 h).*
b.
If an atypical antipsychotic is preferred, consider an agent with low sedation (i.e., risperidone); unless a sedative agent is needed to restore the sleep-wake cycle if there is not response to E.4 (see above).
2.
In patients with extreme psychomotor retardation or catatonic features, in the absence of agitation or psychosis, consider the use of psychostimulant agents (e.g., methylphenidate, dextroamphetamine, or modafinil) or conventional dopamine agonists (e.g., bromocriptine, amantadine, or memantine).