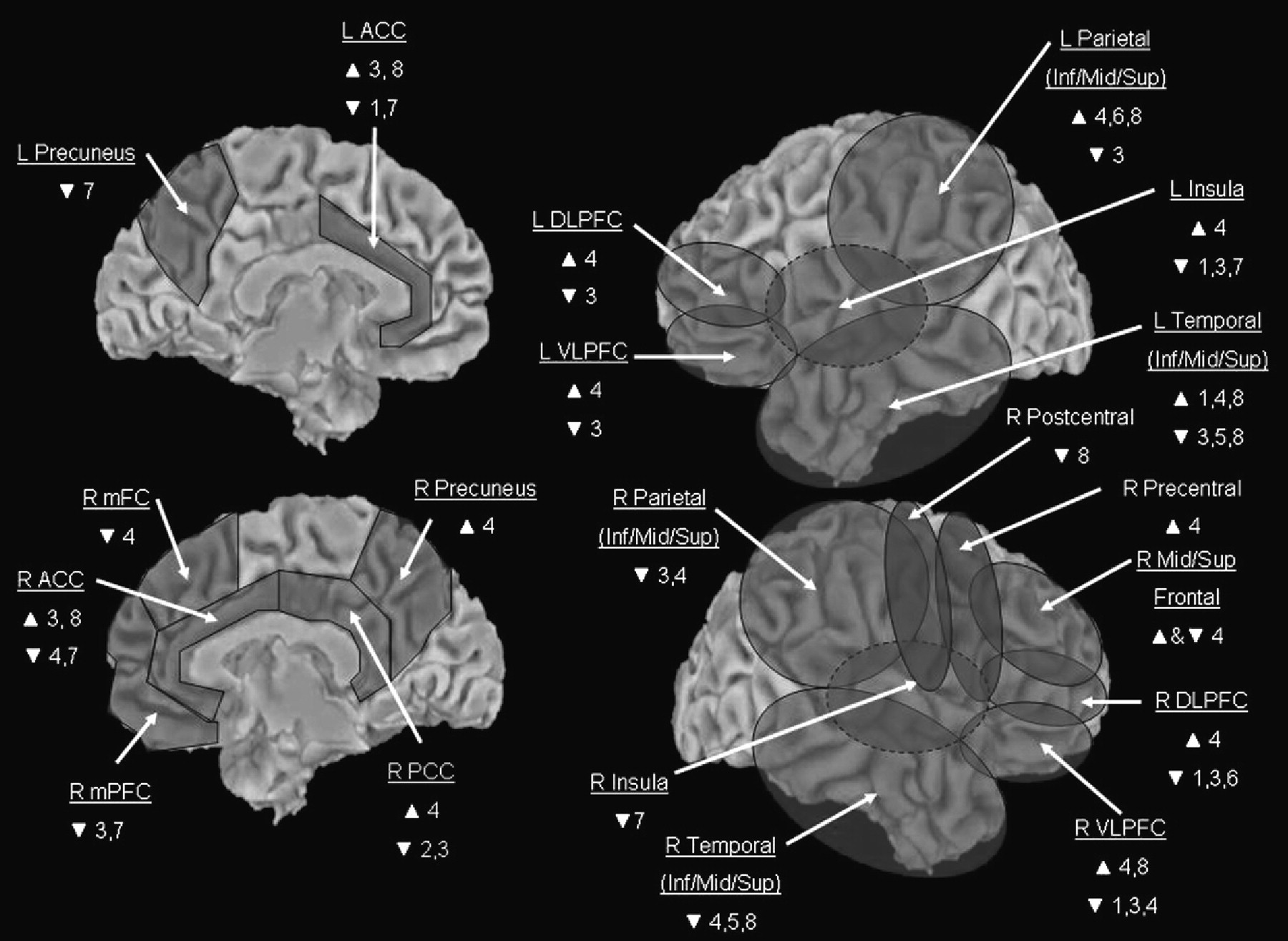
The studies reviewed above, to a varying degree, exhibit procedural strengths that are pertinent to all forms of treatment outcome research, such as the incorporation of formal diagnostic methods, psychometric testing, and behavioural ratings that indicate the degree of symptom severity pre- versus post-treatment. However, additional studies will be required before firm conclusions can be drawn about potential direct causal links between participation in structured psychotherapeutic interventions, such as CBT and IPT, and changes in brain function reflective of improved psychological health. For example, a limitation common to all extant studies involves the relatively small sample size used, which limits the generalizability of the findings. Accordingly, future studies are advised to incorporate larger samples. Additional recommendations for future neuroimaging studies of psycho-therapy outcome are offered below, with the intention of maximizing the validity of future study designs, and capitalizing on the degree to which future studies will be able to definitively address important questions about the neural effects of CBT, IPT, and other structured psychological interventions for mood and anxiety disorders. These recommendations are described in detail below, and additionally outlined in brief in
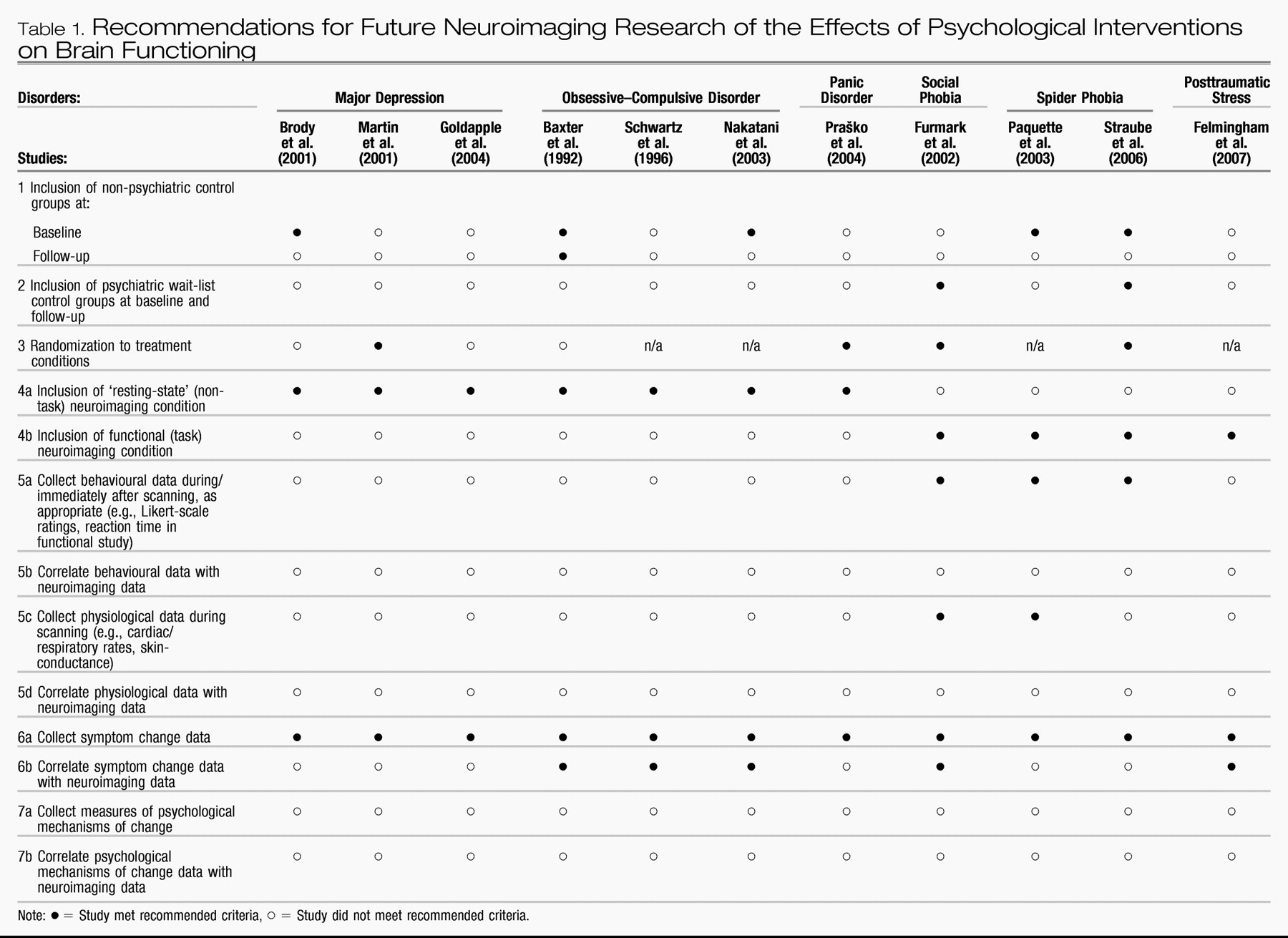
Table 1 for easy reference.
Table 1 also evaluates each of the studies reviewed in this article according to the degree to which it exhibits the recommended characteristics of a methodologically sound neuroimaging study of psychotherapy outcome as advocated herein.
1Recommendations
The first three recommendations listed in
Table 1 are relatively straightforward. The first general recommendation is to include non-psychiatric control groups at pre-treatment for baseline comparison purposes. Only half of the studies reviewed above included a non-psychiatric control group for comparison purposes at pre-treatment (
Baxter et al., 1992;
Brody et al., 2001;
Nakatani et al., 2003;
Paquette et al., 2003;
Straube et al., 2006). These exclusions make interpretation of the findings difficult by prohibiting unambiguous determination of the psychological significance of the distinguishing patterns of brain function demonstrated by clinical participants at baseline. As
Thase (2001) astutely noted, if abnormal brain functioning is not confirmed for clinical participants at baseline (i.e., in comparison with non-psychiatric controls), it precludes sensibility to expect participants' possibly already conceivably ‘normal’ brain function at baseline to ‘normalize’ further with treatment. Equally important, it is prudent for researchers to assess the representativeness of the neuroimaging profiles of their clinical samples at baseline (i.e., relative to previously conducted neuroimaging studies that have compared the same clinical groups to non-psychiatric controls). Non-replication of previously published group differences at baseline may suggest that the sample under investigation is unique, whereas replication would imply that the neuroimaging outcomes occurring with psychotherapy in a given study may be generalizable to the population of individuals with the same psychiatric disorder being investigated.
Second, it is advisable for studies to include a wait-list control group with the psychiatric disorder under investigation, and that an absence of differences in neuroimaging results between the treated and wait-list groups is established at baseline. Only the
Furmark et al. (2002) and
Straube et al. (2006) studies included wait-list control groups, and only the latter explicitly reported whether their treatment and wait-list groups differed with respect to baseline neuroimaging profiles. The inclusion of wait-list control groups diagnosed with the psychiatric disorder being investigated is essential as a methodological control for brain changes that may occur merely in association with repeated testing and/or over time.
For example, particularly in cases where participants have not been acclimatized to the scanning environment itself prior to baseline testing, subsequent scanning sessions may be perceived as more or less stressful than a previous session(s) as a consequence of experiences taking place during the earlier sessions. Moreover, it is possible that state-functional neural images of contrasts between groups of individuals with psychiatric disorders in comparison with controls may unduly reflect state anxiety-arousal engendered by the scanning procedure itself. In other words, although fears about the imaging scanner and scanning environment may be germane to the symptoms that are definitive of various specific psychiatric disorders (e.g., fear of being unable to escape from within the bore of an MRI scanner in an individual suffering from agoraphobia), research questions of interest typically do not directly concern the neural correlates of anxiety reactions associated specifically with brain scanning. Thus the question of whether neural differences between groups might be confounded by state anxiety engendered by the scanning procedure warrants judicious consideration. It therefore seems advisable that participants' level of fear concerning the scanning procedure itself be measured, and potentially used as a regressor of interest on the brain results obtained. This is arguably especially the case for resting-state studies where clinical participants with anxiety disorders may find themselves in a decidedly less ‘restful’ state than control participants during brain scanning. Briefly, a third recommendation, one critical for any study seeking to establish causal significance, is that participants be randomized to the various treatment conditions under study. In less than half of the studies reviewed above were participants randomized to treatment conditions (see
Table 1).
A fourth methodological recommendation is that, whenever possible, investigators should conduct both resting-state and functional neuroimaging analyses at the pre- and post-treatment intervals. Furthermore, it will be important for future studies to examine possible associations between resting-state and functional brain responses in individuals with and without the psychiatric disorders under investigation. None of the previously published studies included both resting-state and functional-experimental neuroimaging conditions.
The inclusion of both resting-state and functional response paradigms is important because the two neuroimaging designs are likely to index different neural facets of clinical psychological presentation. Resting-state studies are important to examine as they may be particularly indicative of the tonic clinical presentation of individuals, and they are conceivably less susceptible than are functional paradigms to experimental demand effects (and by nature practice effects). Additionally, there has been increasing interest in the cognitive neuroscience community in understanding the neural processing underlying the resting or ‘default mode’ state in humans (e.g.,
Fox, Corbetta, Snyder, Vincent, & Raichle, 2006;
Fransson, 2005,
2006;
Grecius, Krasnow, Reiss, & Menon, 2003). Indeed certain investigators envision that this literature may bear significantly on our ultimate understanding of cognition-emotion interactions and of psychiatric symptomatology (e.g.,
Drevets & Raichle, 1998). It is possible that resting-state findings may be most indicative of a general marker of psychopathological disturbance. For example, by the DSM-IV criteria, a symptom bearing on the diagnosis of many forms of affective disorder is that of the individual's perceived difficulty with maintaining his or her concentration and attention, and disturbances in memory also often accompany the diagnosis of mood and anxiety disorders. Differences in resting-state neural function, for example, may be particularly sensitive to these types of general cognitive markers of psychopathology. In studies of this nature, however, it is important that investigators ensure that during the resting-state period psychiatric and non-psychiatric study participants are similarly engaged. For example, psychological processing such as degree of self- versus external-focused attention, frequency of intrusive thought or rumination, and degree of state anxiety or arousal experienced during the resting-state period may differ systematically across individuals with the psychiatric disorder under investigation. Again, in this situation, the experimental conditions may not be parallel in the clinical and control participants, perhaps then posing interpretative barriers on valid inferences about brain activity in the two groups, particularly when such behaviour is not effectively measured (i.e., by self-report). Such measurements may also be pertinent to the valid interpretation of resting blocks interspersed within functional paradigms as well, particularly in cases where resting blocks are frequent and/or lengthy. Given that neural activity occurring during active time points within a functional neuroimaging paradigm is often ascertained as a comparison with neural activity occurring during resting blocks, it is important to verify that clinical and control participants are similarly engaged during the resting portions of the paradigm.
Although resting-state studies are important to conduct, several theorists have proposed that vulnerability to psychopathology is most appropriately studied when participants' dispositions toward experiencing abnormal psychological states are in a ‘primed’ or active state (e.g.,
Ingram et al., 1998). For example, theorists have proposed that vulnerability to depression is conferred primarily when negative self-referential processing is activated during periods of sad-dysphoric mood; current cognitive theories hold that depression-vulnerable individuals may not otherwise appear different from non-psychiatric controls during more euthymic or less affectively-toned states (
Ingram et al., 1998). Thus functional paradigms that prime disorder-relevant psychological-neural processing may be more sensitive to group differences between individuals with mood and anxiety disorders in comparison with controls and, accordingly, neuroimaging paradigms that invoke such processing may be more sensitive to group and pre-post treatment differences as well. Additionally, to the degree that functional tasks hold constant the information processing taking place during scanning within (if not between) clinical and control groups, the neural activations that are observed may be more easily interpretable than those obtained from resting-state studies. Specifically, the unconstrained nature of the psychological processing that participants may be involved in during resting-state studies can serve to delimit the number of plausible interpretations that might ultimately be made about the results that are obtained at a psychological level of analysis.
At the same time, fashioning an experimental paradigm that comprehensively probes all of the psychological processes clinically relevant to a given psychiatric disorder is probably not possible. In other words, the psychological tasks used in neuroimaging studies are likely to measure only certain facets of a disorder, while being less sensitive to other components of an overall clinical presentation. An illustration is available from the posttraumatic stress disorder (PTSD) literature, where a paradigm commonly employed is trauma script-driven imagery, in which participants are scanned while listening to audio-scripts that recount their traumatic experiences. This paradigm consistently provokes re-experiencing symptoms (e.g., sensory flashbacks, intense emotion, and physiological hyperarousal) and/or dissociation in individuals with PTSD, and the neural correlates of these experiences have been studied (reviewed by
Lanius, Bluhm, Lanius, & Pain, 2006). However, whereas this paradigm may effectively index psychological processing that is involved in the re-experiencing and dissociative symptoms of PTSD, it may not be a particularly good indicator of the psychological processing underlying these individuals' emotional numbing symptoms, which nevertheless are arguably just as integral to complex clinical presentations of PTSD (
Frewen & Lanius, 2006).
A fifth recommendation listed in
Table 1 is that researchers collect self-report, other behavioural, and/or other psychophysiological data during or immediately after the neuroimaging scanning. As examples, such data might represent Likert-scale emotion ratings in response to an affect generation paradigm, response times in a vigilance-detection task, or heart-rate or skin-conductance level in response to an arousal manipulation. Not only can such data validate that an expected experimental manipulation took place (i.e., a clinical group responded more strongly to the manipulation than did a control group, or vice versa), but between-participant variability in these data can also be incorporated as regressors of activity in brain regions of interest, as appropriate to the research questions of interest. The majority of the functional neuroimaging studies of psychotherapy outcome conducted to date (
Furmark et al., 2002;
Paquette et al., 2003;
Straube et al., 2006) collected self-report ratings, although none of these studies tested these ratings as predictors of brain activity in their statistical analytic designs (see
Table 1). Specifically, collection of these data respects potential individual differences that may emerge not only between clinical and non-clinical groups but also within each group in terms of neuropsychological response to an experimental paradigm. Therefore, whereas a clinical group as a whole may show differences from a control group, within the clinical group there may still remain a significant level of variability in responsiveness to a particular neuroimaging paradigm. Incorporation of this individual variability in one's modeling of neural responses may account for a greater amount of variance in the neuroimaging signal measured in comparison with a statistical model that solely attributes variability in neural processing to group-level or task-indexed differences (e.g.,
Hopper, Frewen, van der Kolk, & Lanius, in press;
Phan, Taylor et al., 2003;
Phan, Wager et al., 2003).
A sixth recommendation is that neuroimaging data be correlated with symptom severity, including analysis of both treatment responders and non-responders. Several of the previously conducted studies tested correlations between brain responses and psychometric scores that measured psychiatric symptom severity. However, several studies also excluded treatment non-responders from the outcome analysis. In contrast, where symptom change is measured continuously as opposed to dichotomously, such variation in outcome can be correlated with variation in brain response, an analytic strategy that may more feasibly be inclusive of all pre-treatment participants in the post-treatment analysis, a desirable quality on both methodological as well as statistical grounds. It may also be important to measure the degree to which participants' experience psychiatric symptoms during scanning (e.g., level of depressed mood in a depressed participant) rather than only retrospectively (e.g., average level of depressed mood in depressed individual over the previous week/month). Measuring state symptoms in addition to measuring averaged symptoms over a longer duration is important because the effect of the latter may be partly moderated by the degree of the former.
In addition to symptom-based measures, a final recommendation is that investigators incorporate psychometric measures of change on variables theoretically purported to mediate symptom reduction in a given psychotherapeutic intervention. This recommendation would theoretically afford investigators the opportunity to examine the neural correlates of change associated with the mediating psychological variable, in addition to brain changes signifying symptom remission, which may or may not significantly overlap. In other words, while analyses of symptom change alone may primarily reveal overlapping neural correlates of the effects of psychological and pharmacological interventions, as found in several studies reviewed here (
Baxter et al., 1992;
Brody et al., 2001;
Furmark et al., 2002;
Martin et al., 2001), analyses of psychological change may reveal brain changes that are distinctively the result of structured psychological interventions. For example, within CBT interventions for depression, the intention of clinicians is typically to teach participants, among other things, self-monitoring skills (e.g., awareness of negative automatic thoughts) and skills in cognitive reappraisal as means of regulating affective states and altering self-schemas. However, whereas at post-treatment a majority of individuals may be classified non-depressed based on self-report (i.e., survey or clinical interview), participants may nevertheless vary considerably with respect to how effectively they learned (a) the self-monitoring and cognitive reappraisal skills, and (b) the degree to which such learning mediated the depressive symptom reduction they experienced across time (e.g., in comparison with participants treated to symptom remission via pharmacotherapy). If such variation in learning and its mediation of symptom change can be systematically quantified, independently of variation in depressive symptoms (e.g. with a validated psychometric scale, interview, or experimental response measure), pre-post treatment change scores on such a measure can be correlated with pre-post change in brain responses. This kind of analysis would facilitate the identification of brain regions that may be directly associated with and perhaps mediate the increased coping and self-regulatory skills learned in a treatment like CBT, which may indeed not be identical to the neural correlates of symptom remission. In investigations of this nature, future studies may choose to follow the statistical analytic methodology for testing mediation outlined by
Baron and Kenny (1986).
Finally, previous studies have exclusively focused on describing the neural correlates of CBT and IPT relative to either PT or no-treatment. In contrast, no published neuroimaging studies have compared the effects of different structured psychological interventions, or even compared the effects of structured psychological interventions like CBT and IPT to less structured psychological interventions (e.g., supportive therapy). Thus, for studies seeking to identify the neural correlates of specific therapeutic factors, psychotherapy control groups will need to be included. A common research strategy along these lines is to compare the efficacy of a structured psychological intervention to a less structured one as a means of controlling for non-specific aspects of psychotherapy (e.g., positive therapeutic alliance). Rather than including forms of psychotherapy previously shown to be sub-optimal as treatment for a particular mood or anxiety disorder, however, an alternative means of assessing the role of the therapeutic alliance is as a moderator or mediator of treatment outcome
within an already empirically-supported structured psychological intervention. In this way, the possible direct and indirect neural effects of participation in a healing relationship, such as that engendered by certain forms of psychotherapy, can be probed using neuroimaging designs without requiring inclusion of a ‘placebo-based’ psychological treatment. Examination of the effects of the therapeutic alliance may be a particularly productive research strategy in studies of the effects of psychotherapy for psychiatric disorders that are, arguably, inherently relational (e.g., atypical depression, social anxiety disorder, posttraumatic stress disorder associated with interpersonal violence and/or abuse). Furthermore, studies in social-cognitive neuroscience are beginning to uncover how perceived level of social support modulates neural responses to anxiety (e.g., threat of shock;
Coan, Schaefer, & Davidson, 2006) and social exclusion (
Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007). These methods may be profitably adapted to future studies of the strength of the therapeutic relationship as a moderator or mediator of the neural changes taking place over effective psychological treatments for mood and anxiety disorders.
In summary, the studies reviewed above lay the groundwork for additional methodologically rigorous and theoretically sophisticated tests of the effects of psychological interventions on brain function to be conducted in the future. As such, the present juncture is an opportune time to identify standards for the conduct of future investigations of the neural effects of structured psychological interventions for mood and anxiety disorders.
Table 1 lists the above provisional recommendations for future studies seeking to test the effects of psychotherapeutic interventions on brain functioning in different psychiatric disorders (see also
Beutel, Stern, & Silbersweig, 2003, for a set of general recommendations regarding research collaborations conducted between neuroscientists and psychotherapists).