Our series of studies has capitalized on the population-based, longitudinal birth cohort study of the Cardiovascular Risk in Young Finns (
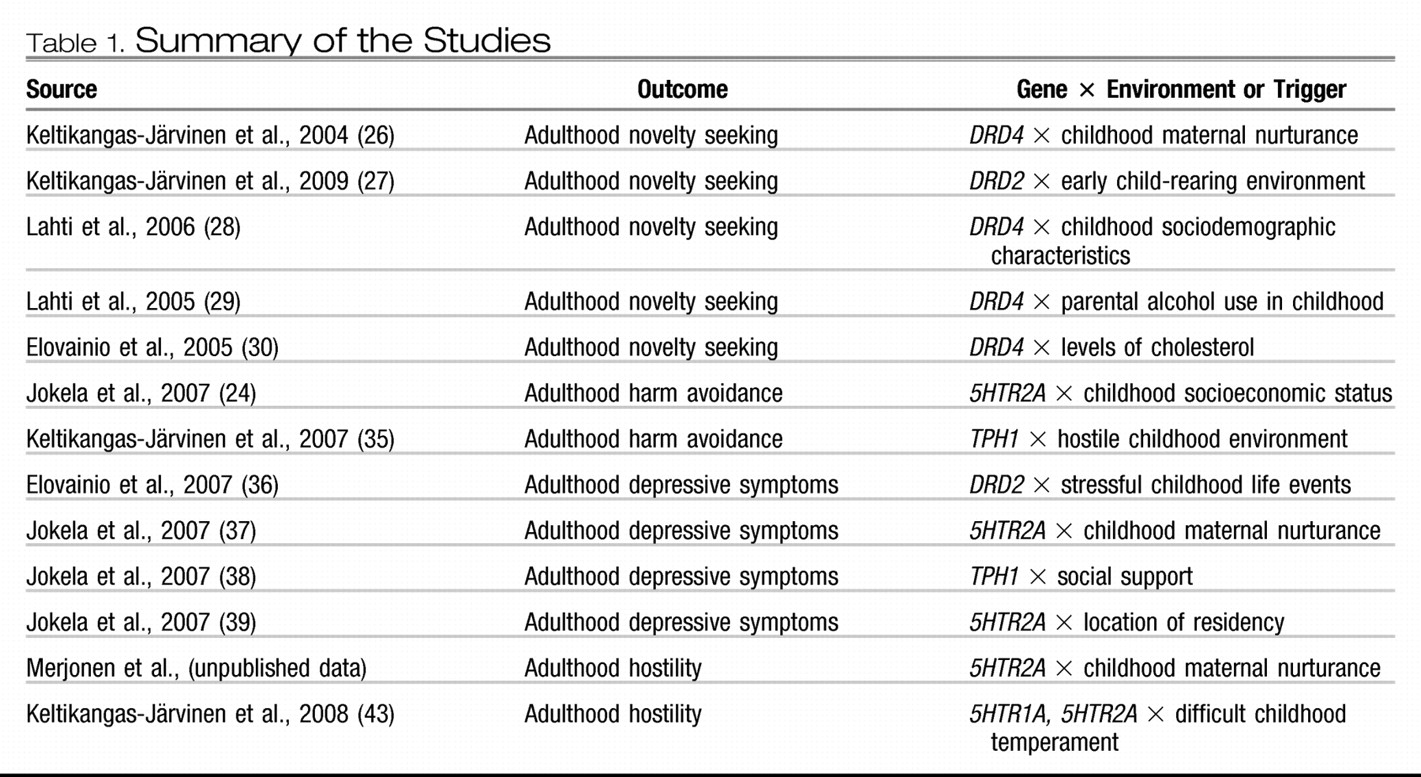
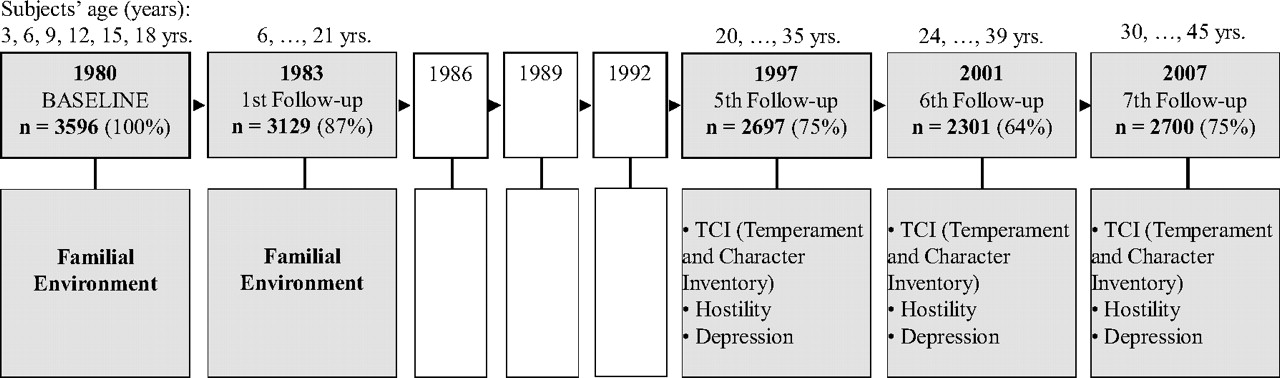
Table 1). In this study, a representative sample of 3,600 healthy subjects from six age cohorts (aged 3, 6, 9, 12, 15, and 18 years at the baseline) have been followed over 27 years since their childhood and monitored in eight study waves resulting in a huge reservoir of somatic, psychological, behavioral, and environmental predictors and somatic and psychological outcomes (
23) (
Figure 1).
Nature versus nurture in temperament
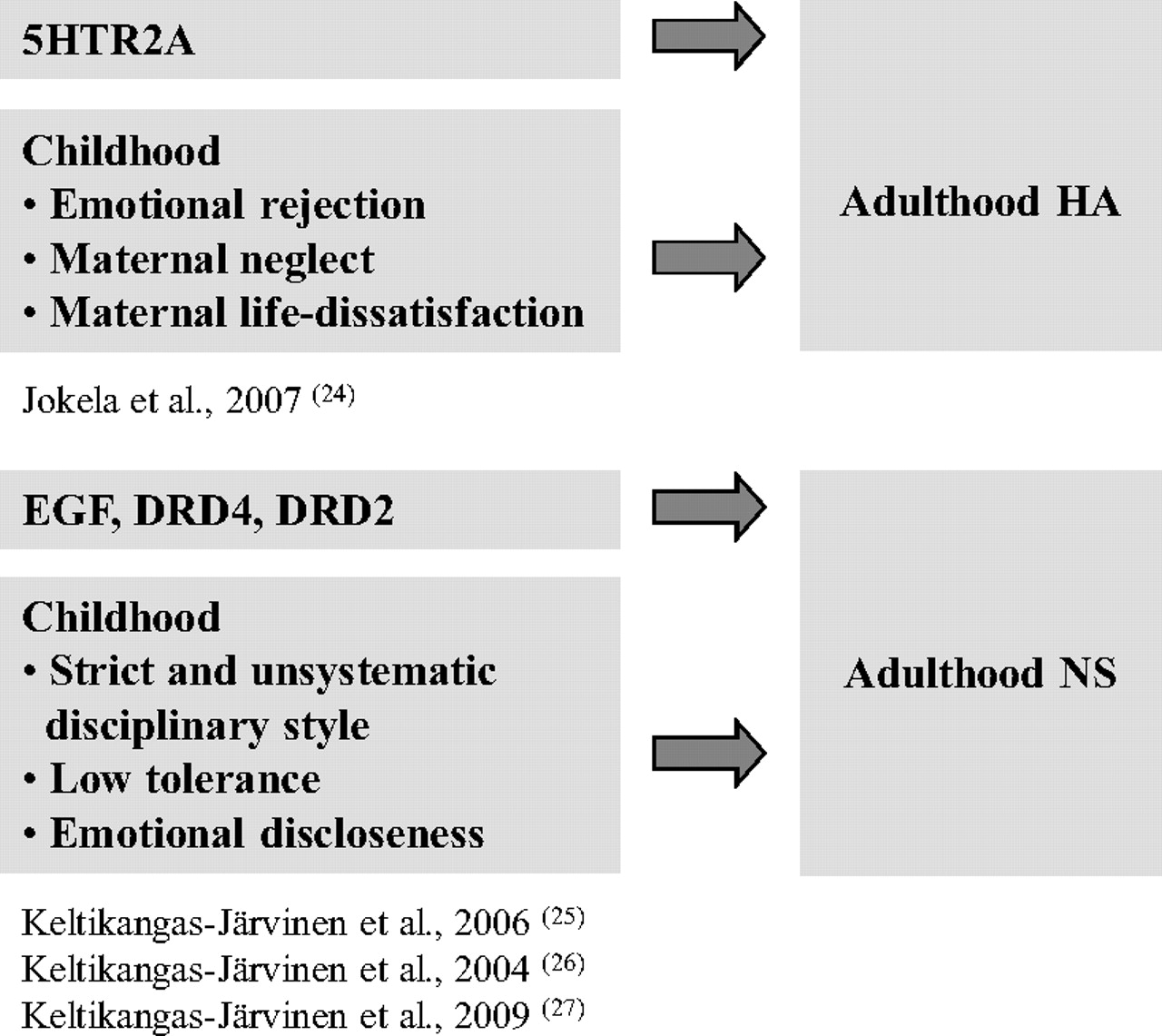
We found both genetic and environmental main effects on temperament (
24–
27) (
Figure 2). An interplay between genetic background and childhood environmental circumstances in the development of adult NS and HA existed, too. First, our findings suggest that the polymorphic variations of two central candidate genes of dopaminergic system, i.e., in
DRD2 and
DRD4, may moderate the influence of childhood environment on adulthood NS. This effect was first shown in a group of oversampled subjects (subjects with constantly extremely high or low NS) derived from the Cardiovascular Risk in Young Finns. In a hostile childhood environment, characterized by an emotional distance between the child and the mother and the mother's strict disciplinary style, the individuals carrying two- and five-repeat alleles of the
DRD4 gene had a significantly greater risk to constantly score extremely high in NS compared with the individuals not carrying any of these alleles. A more favorable childhood environment, instead, predicted a low level of NS in adulthood, even in the presence of this very genotype (
26).
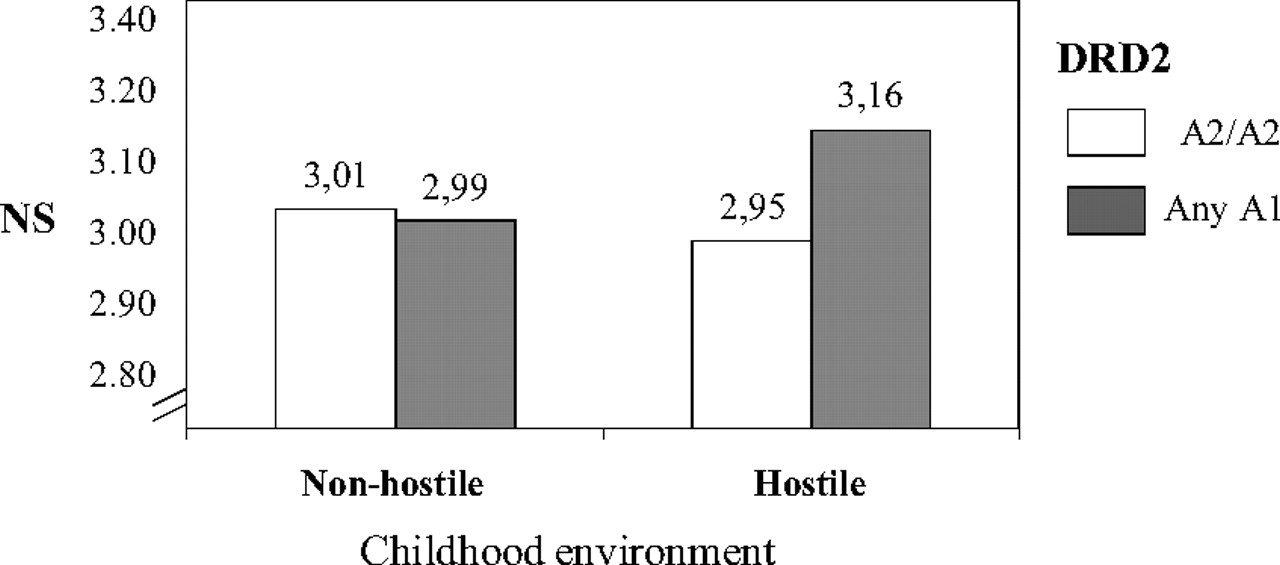
This finding was repeated in the total Cardiovascular Risk in Young Finns sample and with a variant of another gene polymorphism related to dopaminergic transmission, i.e.,
DRD2. In a highly hostile child-rearing environment, especially in terms of disciplinary style, the A1 allele carriers of the Taq1A variant of the
DRD2 gene had higher scores for NS in adulthood than A2/A2 genotype carriers. When the childhood environment was emotionally positively tuned, the genotype had no effect on NS (
27) (
Figure 3).
In addition to emotional atmosphere, the
DRD4 two- or five-repeat alleles interacted with childhood socioeconomic circumstances, residential setting, and parental alcohol use. In the carriers of this gene variant, high maternal education, high annual household income, and an urban residential setting in one's childhood increased the likelihood of scoring high on NS in adulthood. This was true after controlling for the effects of emotional relationships between the child and the mother. When maternal education and household income were low or the family resided in a rural setting, no differences between extreme high and extreme low NS groups were found (
28). Further, in the presence of high paternal alcohol consumption, the two- or five-repeat alleles of the
DRD4 were associated with a high level of NS, whereas no association was found in the presence of low alcohol consumption (
29).
Somatic parameters modified an effect of the
DRD4 polymorphism on NS, too. The two- or five-repeat alleles were associated with high NS in subjects with high levels of cholesterol but not in those with low cholesterol levels. This finding remained after adjustment for the apolipoprotein E polymorphism (
30).
Second, we studied gene × environment interactions in the development of HA by using the T102C variant of the serotonin receptor 2A (
5HTR2A), and A218C and A799C haplotypes of the tryptophan hydroxylase 1 gene (
TPH1) as markers of the serotonin functioning. The T102C variant of the
5HT2RA polymorphism has been associated with the expression of the gene and with the binding potential of serotonin 2A receptors (
31), and it has been considered as a candidate gene polymorphism for many psychiatric disorders, e.g., depression (
32) and anxiety (
33).
TPH1 is the rate-limiting enzyme for serotonin biosynthesis. It regulates serotonin levels and influences behaviors that are controlled by serotonin. Neurogenetic studies have mostly focused on A218C and A799C haplotypes of TPH1, and those polymorphisms have been shown to be associated with depression and suicidal behavior (
34).
We showed that the T102C polymorphism of the
HTR2A gene was directly associated with HA (
24) and moderated the effect of childhood familial socioeconomic status (SES) on adulthood HA. The carriers of the T allele scored low on HA in adulthood if their familial SES was high in childhood or adolescence, whereas no association was observed among those with low familial childhood SES. This finding suggests that the T allele carriers may be more sensitive to environmental effects than the C/C carriers (
24).
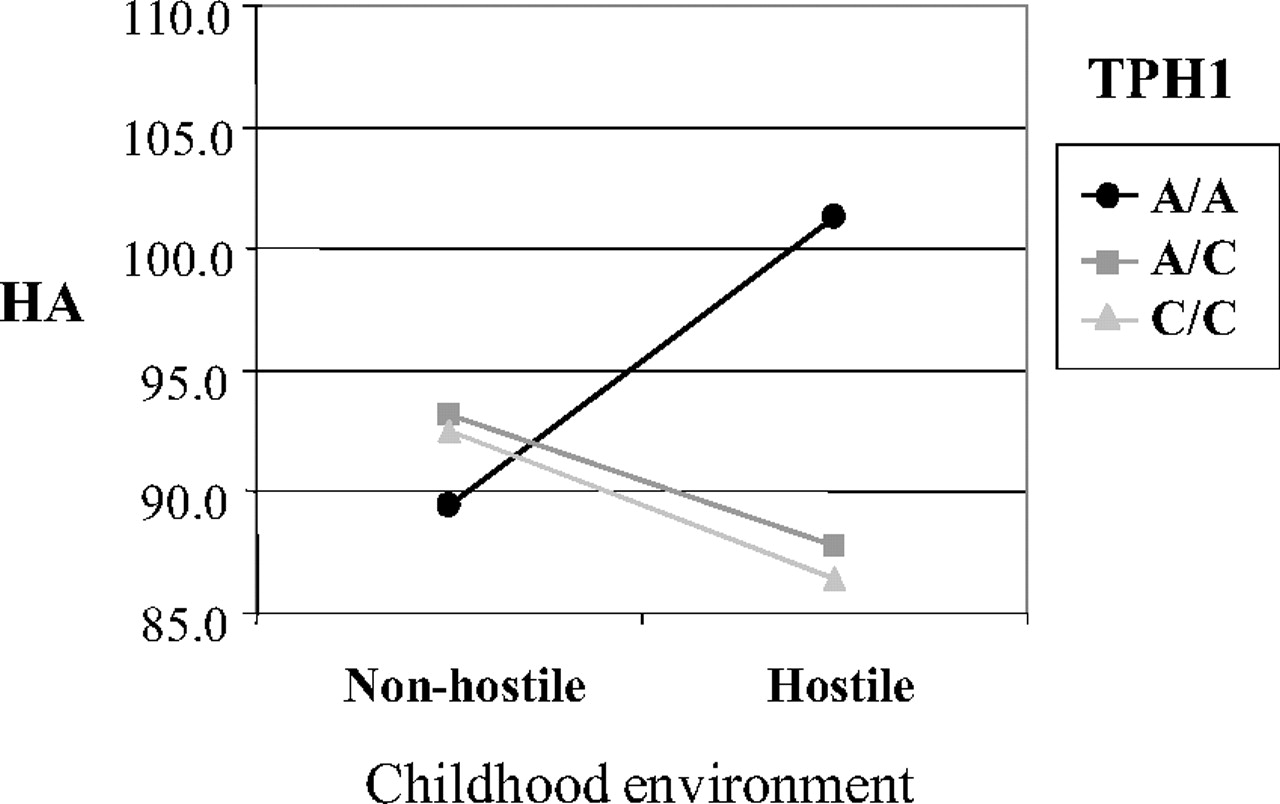
The joint effect of environment and genetic background was true with
THP1 also. The A/A haplotypes of the
TPH1 intron 7 A218A and A779C polymorphisms predicted a high level of adulthood HA in the presence of a hostile childhood environment as defined in terms of emotional rejection and inconsistent discipline, whereas no environmental effect was identified among C/C carriers (
35) (
Figure 4).
All the above gene × environment interactions effects on NS and HA were repeated at two or three different study phases of the Cardiovascular Risk in Young Finns study (samples highly overlapping but not identical) 4 or 5 years apart.
Our most recent unpublished finding suggests an interaction of childhood nurturing environment and the T102C polymorphism of 5HTR2A on social attachment, as measured with the attachment subscale of RD and related measures of adult attachment styles. T/T genotype carriers were found to benefit from a favorable maternal nurturance in childhood and adolescence by showing greater temperamental attachment in adulthood, whereas an unfavorable maternal nurturance in childhood induced a lower attachment security in adolescence among them. In C allele carriers, a quality of maternal nurturance was not associated with later social attachment.
Nature versus nurture in depression
Our findings have also suggested moderating roles for the
DRD2,
TPH1, and
5HTR2A in a development of depressive symptoms. We found a weak overall association between childhood negative life events and adulthood depressive symptoms. This association was stronger among those who carried the A/A genotype of the Taq1A polymorphism of
DRD2 and weaker among those with other genotypes (
36).
We also found that the T allele carriers of the T102C polymorphism of
5HT2RA who had experienced high maternal nurturance in childhood or adolescence expressed lower levels of depressive symptoms in adulthood, compared with their C/C genotype counterparts, suggesting that the T allele carriers were more able to benefit from the protective aspects of the environment (
37). Further, we found that low social support predicted depressive symptoms more strongly in individuals carrying A alleles of the
TPH1 than in those with other alleles (
38).
Finally, the T102C polymorphism of the
5HT2RA was shown to moderate the effect of urban/rural living conditions on depressive symptoms. In T allele carriers, urban or suburban residency was associated with lower depressive symptoms, whereas living in remote rural areas predicted a high level of depressive symptoms in them. No correlation was observed among individuals carrying the C/C genotype (
39).
Nature versus nurture in hostility
A nonsupportive and conflictual childhood family environment has been shown to contribute to development of hostility (
40), whereas twin studies suggest that hostility also has a genetic component (
41). Hostility and difficult temperament share the same elements (
7), and hostility has been suggested to be mediated by the serotonergic system (
42).
Given these previous findings, we studied the modifying effects of the 5HTR1A and 5HTR2A gene polymorphisms in the association between childhood maternal behavior (quality of nurturance) and adulthood hostility. Among the carriers of the T/T and T/C genotypes of 5HT2RA, nurturance predicted hostility, whereas no association among C/C carriers was observed (unpublished data).
In addition, the T102C polymorphism was also shown to moderate the association between childhood hyperactive temperament and adulthood hostility in men (
43). T/T genotype carriers, who were rated as hyperactive in childhood by their mothers, expressed higher levels of hostility in adulthood. The same was not true among C allele carriers. A child's hyperactive temperament might create a hostility-prone environment, i.e., a child's own behavior contributes to an unfavorable parent-child relationship and the T/T gene variant selects the children who are most sensitive to this detrimental influence.