Apathy is defined as diminished motivation not attributable to decreased level of consciousness, cognitive impairment, or emotional distress.
1 Depression involves considerable emotional distress, evidenced by tearfulness, sadness, anxiety, agitation, insomnia, anorexia, feelings of worthlessness and hopelessness, and recurrent thoughts of death.
2 Traditional diagnostic nomenclature and measures of depression, including the DSM-IV
2 and the Hamilton Rating Scale for Depression (Ham-D),
3 treat apathy as an aspect of depression, but it may be a discriminable clinical construct that requires a different approach to treatment in some patients.
4–7Marin et al.
7 evaluated patients with Alzheimer's disease (AD), stroke, and major depression by using the Apathy Evaluation Scale
8 and the Ham-D. They found that the relationship between apathy and depression varied among diagnostic groups. Apathy and depression were significantly correlated within groups, although absolute levels of apathy and depression varied considerably. Alzheimer's disease patients frequently had high apathy and low depression scores, whereas patients with left hemisphere stroke or major depression frequently had high depression with low apathy scores. Patients with right hemisphere stroke composed the only group that had equal levels of the two symptoms, and it was the only group in which apathy and depression were not correlated. The authors suggested that apathy and depression are clinically distinct neuropsychiatric syndromes.
Neurodegenerative disorders facilitate the study of apathy and depression because they produce demonstrable brain pathology and are frequently associated with neuropsychiatric symptoms.
5,9–13 The Neuropsychiatric Inventory (NPI)
14 was designed to assess neuropsychiatric symptoms in dementing illnesses and includes separate subscales for apathy and depression. In two previous studies, we found that patients with frontotemporal dementia (FTD) and progressive supranuclear palsy (PSP) could be discriminated from patients with AD by their more severe apathy and relatively less depression.
9,11 We hypothesized that apathy or lack of emotion may be different from depression and speculated that the two symptoms may be produced by different neuroanatomical or neurochemical substrates and would be differentially manifested in a variety of neurologic disorders.
METHODS
Subjects
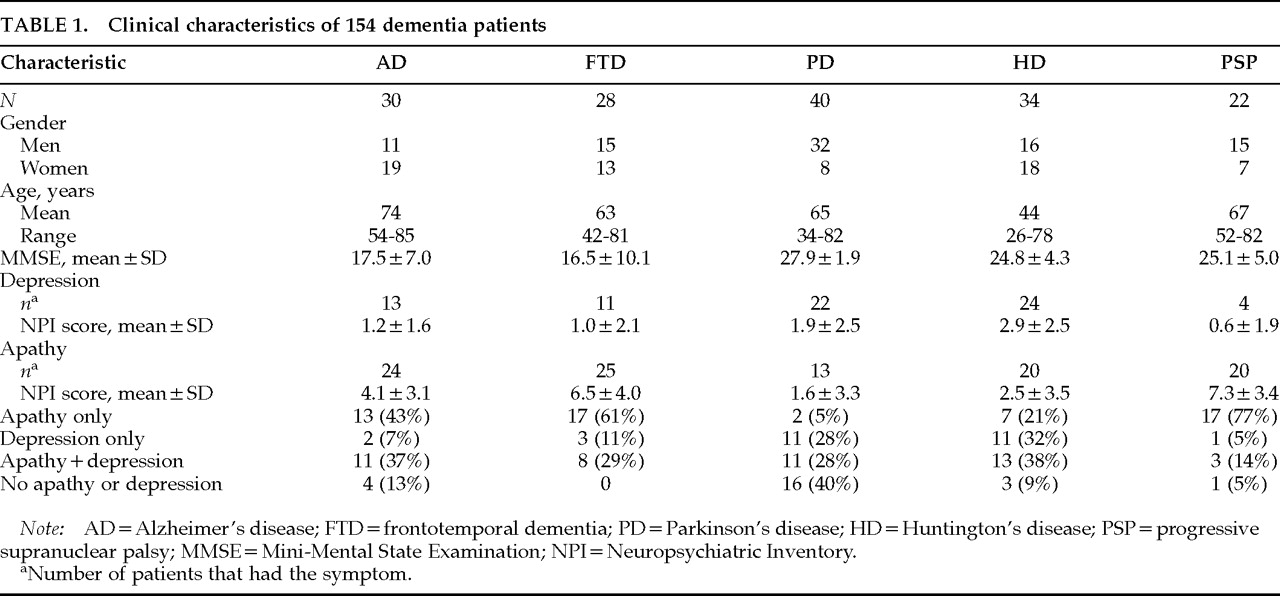
A total of 154 patients were seen at four academic centers. Study participation required informed consent from both the patient and the caregiver.
Thirty AD patients and 28 FTD patients presented to a dementia clinic at the University of California at Los Angeles, University of California at Los Angeles-Harbor Medical Center, or West Los Angeles Veterans Affairs Medical Center. All 30 AD patients and 22 of the FTD patients have been previously described.
9 Probable AD was diagnosed on the basis of criteria established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA).
15 Frontotemporal dementia was diagnosed on the basis of criteria developed by the Lund and Manchester groups.
16Forty Parkinson's disease (PD) patients were referred to a movement disorders clinic at the University of California at Los Angeles for pallidotomy surgery due to the on-off syndrome, intractable tremor, or bradykinesia on maximum doses of anti-PD medications. Patients were screened by two neurologists and a neurosurgeon. Inclusion criteria included a history indicative of idiopathic PD, including two of the four cardinal signs (rest tremor, rigidity, akinesia/bradykinesia, and gait disturbance/postural instability) and a clear history of responsiveness to levodopa.
Twenty-two patients with PSP were seen at a National Institute of Neurological Disorders and Stroke (NINDS) outpatient clinic and have been previously described.
11 All PSP patients met the Blin criteria for probable PSP.
17,18Thirty-four Huntington's disease (HD) patients presented to a specialty clinic at the University of California, San Diego, and all had the typical choreiform movement disorder plus at least one first-degree relative who also had HD.
Patients were screened for chronic mental illness, head trauma, cerebrovascular disease, extrapyramidal disorders other than those described, vitamin deficiency, hypothyroidism, syphilis, and other medical conditions. Not all patients with HD, PSP, and PD had sufficient memory impairment to meet the DSM-IV criteria for dementia; however, they all had degenerative brain diseases as defined by their clinical diagnoses. Our primary focus was not on the level of dementia, but on the relationship of apathy and depression. Secondary analyses examined the relationship of these symptoms to cognitive impairment.
Assessment
Behavioral data were collected during caregiver interviews using the NPI. This instrument assesses 10 behaviors occurring in dementing illnesses, including delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, and aberrant motor behavior (including pacing, rummaging, and compulsions). A frequency rating (1–4) multiplied by a severity rating (1–3) produces a subscale score for each behavior, and summation of subscale scores produces the total NPI score. Another approach to analysis was also used in which we assigned 0 to mean absence and 1 or more to mean presence of the symptom. The NPI has been shown to be valid when compared with a variety of other diagnostic approaches and to have high interrater and test-retest reliability.
14 A training videotape and scripted questions were used to ensure cross-site reliability.
The apathy subscale includes items such as showing loss of interest, lacking motivation, less spontaneous, less affectionate, less enthusiastic, lacking in emotions, and not caring about doing new things. The depression subscale includes items such as sad, depressed, tearful, in low spirits, feeling like a failure, hopeless about the future, feeling like a burden, and wishing for death. The depression subscale has no items related to apathy and has been shown to correlate strongly with the Hamilton depression scale.
14 The apathy subscale has no items related to sadness.
Cognitive function was assessed with the Mini-Mental State Examination (MMSE).
19 A perfect score on the MMSE is 30, and decreasing scores indicate more severe cognitive impairment. The NPI and MMSE were generally administered on the same day.
Data Analysis
The two central issues of the study were 1) how these two symptoms were related to each other and 2) whether or not their relationship was consistent across different dementia syndromes. Spearman correlations were determined for all 10 of the NPI subscale scores and the total MMSE score. A separate matrix was generated for each of the five dementia groups and for the total combined group. A Kruskal-Wallis one-way analysis of variance (ANOVA) on the mean subscale scores was used to determine group differences in the severity of depression and apathy. Analyses are exploratory, and a 0.5 level of significance was accepted as meaningful.
RESULTS
Clinical characteristics of the 154 patients are presented in
Table 1. There were almost equal numbers of men and women in the FTD and HD groups, but there were more women in the AD group and more men in both the PD and PSP groups. The AD group was on average about 10 years older than the FTD, PD, and PSP groups and 30 years older than the HD group. MMSE scores were similar in AD and FTD, and these two groups had MMSE scores 10 points lower than the PD, HD, and PSP groups.
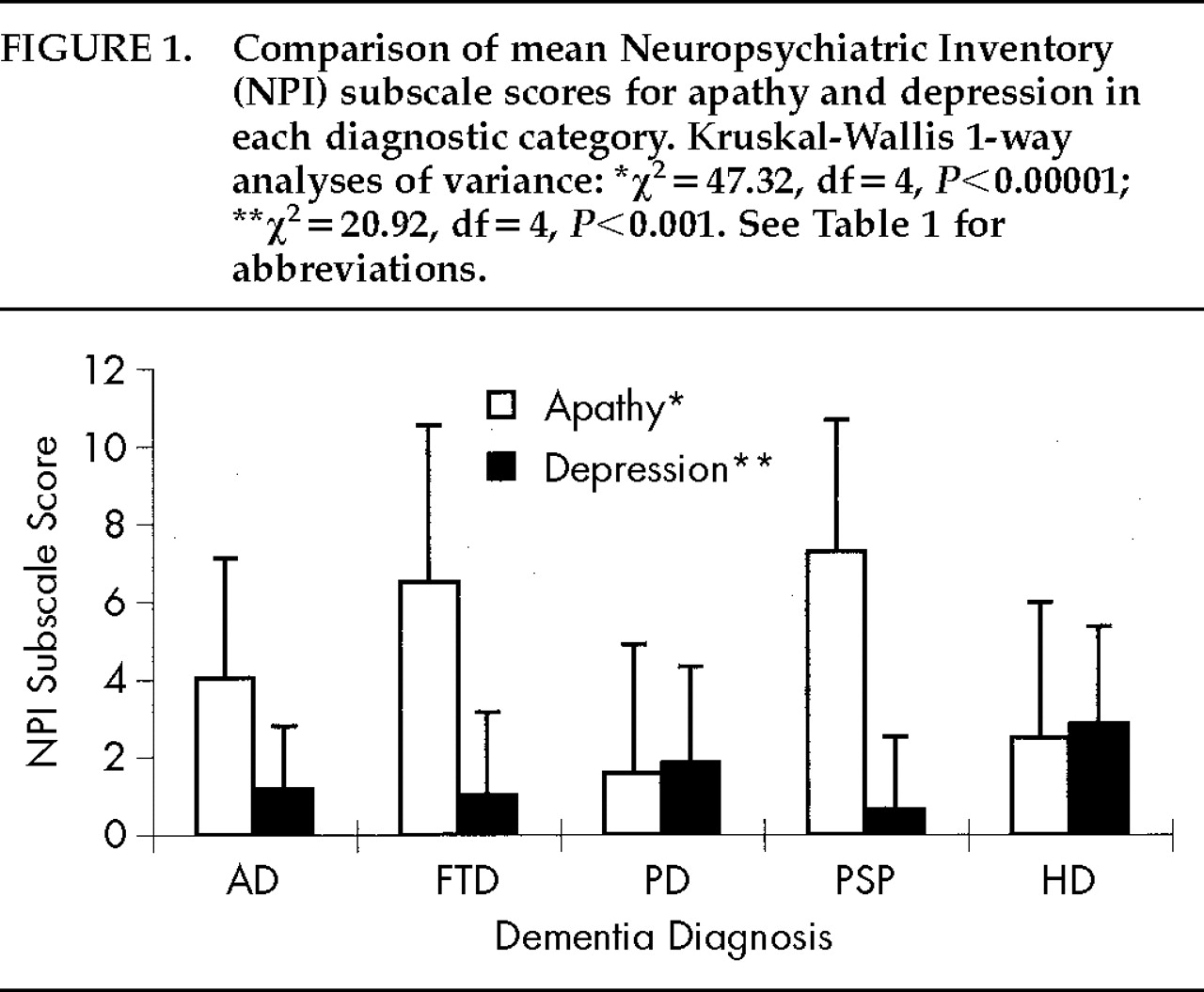
The severity of apathy and depression differed significantly among groups (
Table 1). A Kruskal-Wallis one-way ANOVA was used to compare mean NPI subscale scores among groups. Significant differences were found across the four groups, with PD and HD patients having the least severe apathy and the most severe depression (
Figure 1).
The frequency of apathy and depression and the relationship between the two symptoms varied across diagnostic groups. A large number of AD patients and even more FTD and PSP patients had apathy without depression, whereas many PD and HD patients had depression without apathy. All diagnostic groups had more patients with one or the other symptom than with both symptoms. The disparity between apathy and depression was particularly dramatic in patients with PD and PSP. Few PD patients had apathy alone (
n=2; 5%) compared with the number who had depression alone (11; 28%) or depression plus apathy (11; 28%), and few PSP patients had depression, but they had a high frequency of apathy. Of 22 patients with PSP, 17 (77%) had apathy alone,
11 1 had depression, and 3 had depression plus apathy (
Table 1).
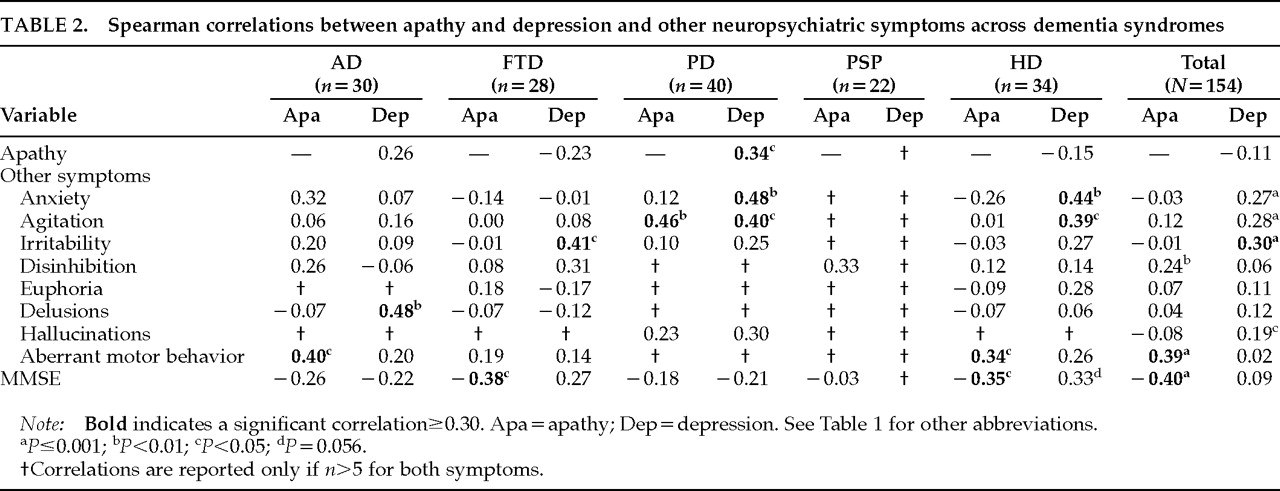
Apathy did not correlate with depression in the combined sample of dementia patients (
r=–0.11,
P=0.18). There was a nonsignificant negative correlation in the FTD (
r=–0.23,
P=0.23) and HD (
r=–0.15,
P=0.40) groups, and there was a nonsignificant positive correlation in the AD group (
r=0.26,
P=0.16). The PD group was the only dementia group to show a significant correlation between the two symptoms (
r=0.34,
P=0.03;
Table 2). Thus, the pattern of correlation between apathy and depression differed across diagnostic groups, and the presence of one did not predict the presence of the other.
The NPI measures eight neuropsychiatric symptoms in addition to apathy and depression. Some of these symptoms correlated with apathy and other, different symptoms correlated with depression. In the total sample, apathy correlated with disinhibition and aberrant motor behavior, and depression correlated with anxiety, agitation, irritability, and hallucinations (
Table 2).
Apathy was correlated with lower cognitive function as measured by the MMSE in the total sample (r= –0.40, P<0.0001), in the FTD group (r= –0.38, P<0.05), and in the HD group (r= –0.35, P<0.05). The AD and PD groups had a trend toward lower cognitive function with increased apathy, and the PSP group had no correlation between apathy and cognitive function. Apathy was not correlated with advancing age.
Depression had no significant correlation with MMSE scores in the combined sample. In AD and PD, there were nonsignificant trends toward a relationship between depression and lower cognitive function. FTD and HD patients showed nonsignificant trends toward relationships between depression severity and better cognitive function, and there were too few PSP patients with depression to support a meaningful analysis.
DISCUSSION
Apathy is highly prevalent in neurodegenerative diseases,
5,9–13 and this study supports and extends findings from the few previous studies addressing the issue of apathy and depression as independent clinical phenomena.
5,7,11,20 Unlike the Ham-D used in many previous studies, the depression subscale of the NPI includes no apathy-related items, and the apathy subscale does not have depression-related questions. We found no correlation between apathy and depression in 154 patients with five neurodegenerative disorders. Furthermore, the two symptoms were associated with other neuropsychiatric symptoms in dissimilar ways. Apathy was associated with disinhibition and aberrant motor behavior; depression was associated with anxiety, agitation, irritability, and hallucinations. Apathy and disinhibition may seem like mutually exclusive behaviors, but both are mediated by frontal lobe systems and they are frequently seen together, especially in patients with FTD. Also, the problem behaviors that lead to management difficulties or reduced functional capacity may be primarily related to frontal lobe dysfunction, and previous studies have found that apathy is associated with these symptoms.
21,22In this and previous studies, apathy was significantly correlated with increased cognitive impairment (as measured by the MMSE) in AD and PD,
5,13,20,23 but not in PSP
11 or HD.
22 The relationship between depression and cognitive function in dementia is controversial. Some studies have found an association between depression and more severe cognitive impairment,
24 but others have not.
25 We found no consistent relationship between depression and cognition in the total sample or in individual diseases. However, the MMSE does not adequately test frontal lobe function, which is frequently impaired in many dementing disorders, and does not provide a valid measure of this part of cognitive decline.
The relationship between apathy and depression appears to be disease-specific. AD, FTD, and PSP had more prevalent and severe apathy, whereas PD and HD had more prevalent and severe depression. PSP patients showed the most frequent and severe apathy and the least depression. One previous study that used different measures of apathy and disease severity found that HD patients were more severely apathetic than AD patients.
22Anatomical localization of regional dysfunction associated with apathy and depression appears to overlap considerably. Depression is reported to be more frequent when focal lesions are anterior and left sided.
26 This finding is similar but not identical to findings from metabolic studies in younger patients showing that major depression is associated with frontal, and especially left frontal, hypometabolism.
27 Parkinson's disease with depression is associated with reduced glucose metabolism in the inferior frontal
28 and medial prefrontal cortex.
29 Paralimbic areas such as orbital-inferior prefrontal and temporal cortex have also been implicated in depression in patients with HD.
30 Apathy results from damage to medial prefrontal, anterior cingulate, and anterior temporal paralimbic areas, especially the amygdala and related subcortical structures.
31–33 Apathy in AD patients has been correlated with frontotemporal hypoperfusion in one study
23 and with right temporoparietal hypoperfusion in another.
21 Apathy may also occur with strokes that involve the posterior limb of the internal capsule.
20 Apathy has only recently been the focus of investigation, and there may be several forms of apathy mediated by a network of related structures. The anatomical correlates of apathy require further investigation, but the observations here suggest that the frontal regions involved in FTD and the diencephalic structures affected in PSP are particularly relevant.
Some of the overlap reported between depression and apathy may be due to the use of depression instruments that contain apathy items. Another possible explanation is that similar circuits could be involved in both symptoms but could differ in degree of neurotransmitter involvement among different disorders. Serotonergic agents frequently relieve depression but may increase apathy, whereas dopaminergic agents may relieve apathy
34,35 but are ineffective as antidepressants; in acetylcholine-deficient diseases such as AD, a cholinomimetic agent may be useful in reducing apathy, but it does not affect mood.
36 One potential explanation for the differential therapeutic responses of depression and apathy is that depression results when an imbalance in paralimbic neurotransmitter function results in excessive negative emotion, whereas apathy occurs when the cortex is functionally disconnected from relevant paralimbic input.
Limitations and strengths of this study should be recognized. First, behavioral assessment was a primary goal in the collection of these data, but the specific hypotheses presented here were not prospectively stated and the analyses are post hoc. Second, depression and apathy are subjective human experiences, and caregiver informant strategies employed by the NPI may not be entirely accurate—although there is evidence to suggest that caregivers are able to provide valid ratings of depression and apathy.
8,37 Third, the PD, HD, and PSP patients had significantly less cognitive impairment, which could account for some of the differences that we described as disease-specific. Fourth, psychotropic medications may cause apathy and depression, and we cannot ensure that some of our patients were not taking them; however, these assessments were generally done on the initial visit prior to drug therapy. Finally, we do not have autopsy data to confirm our diagnostic accuracy.
The study is strengthened by the presence of a large group of patients with neurodegenerative disorders and a wide variation in the clinical and pathological expression of their illnesses, which allows us to draw conclusions about the relationship between apathy and depression in the presence of differentially impaired neural pathways. In addition, the use of a reliable and valid instrument that separates the two constructs allows for a meaningful comparison.
We conclude that apathy is common in neurodegenerative diseases and is separable from depression. It may be treatable with dopaminergic or cholinergic medications, and its presence should be sought during the routine course of a neuropsychiatric evaluation. Apathy should not be ascribed to depression, and the diagnosis of depression should be based on symptoms of sadness and feelings of helplessness, hopelessness, and worthlessness.