Depression, affect incontinence, and fatigue are the three most common psychiatric disturbances in multiple sclerosis (MS), and they have been relatively neglected in treatment studies.
1 Rates of depression in MS patients are reported in the range of 25% to 50%, and suicide rates are higher than in the normal population.
2 Various authors have assessed point prevalence of depression in MS patients, reporting 32.5% suffering from overt depression or 14% suffering from major depression. Sadovnik et al.
3 reported a 50.3% lifetime risk of depression in MS patients.
Duration and severity of depression in MS patients is not different from major depression in psychiatric patients with no comorbid neurological conditions.
4 Minden and Schiffer
1 reviewed affective disorders in MS and published their recommendations for clinical research in 1990. They stress that despite the frequency of depression among patients with MS, few received adequate treatment. The use of tricyclic antidepressants resulted in dropout rates of up to 42% because of intolerable side effects.
5 In addition, patients who completed trials with tricyclic antidepressants were unable to tolerate doses higher than 125 mg daily, well below the reported effective dose for the treatment of depression.
5 Thus, it has been recommended that antidepressants with the fewest sedative, hypotensive, and anticholinergic side effects should be preferred in depressed MS patients.
6Because depression adversely affects quality of life and rehabilitation efforts, and indirectly results in higher suicide rates in MS patients,
1–3 its management is of importance. The reversible inhibitors of monoamine oxidase isoenzyme A (RIMA) are a new class of antidepressants developed and investigated in the last decade. The most widely researched and used compound of this class is moclobemide, which has antidepressant efficacy comparable to the older tricyclics and newer generation antidepressants but with superior safety to tricyclics and classic monoamine oxidase inhibitors.
7The use of RIMA-type compounds is especially tempting in MS patients because these compounds have the advantage of a preferable side effect profile and they also have been shown to enhance cognitive performance and psychomotor functioning.
8,9 The aims of the present study were to evaluate the effect of moclobemide treatment in depressed MS patients and to determine the safety profile and adverse events in response to moclobemide treatment.
METHODS
Subjects
MS patients reporting depressive symptomatology of recent onset (within the previous 2 weeks) were interviewed with the Structured Clinical Interview for DSM-IV, Depression (Hebrew version). Patients diagnosed with major depression (single and/or recurrent episode) according to DSM-IV criteria were considered for the present study.
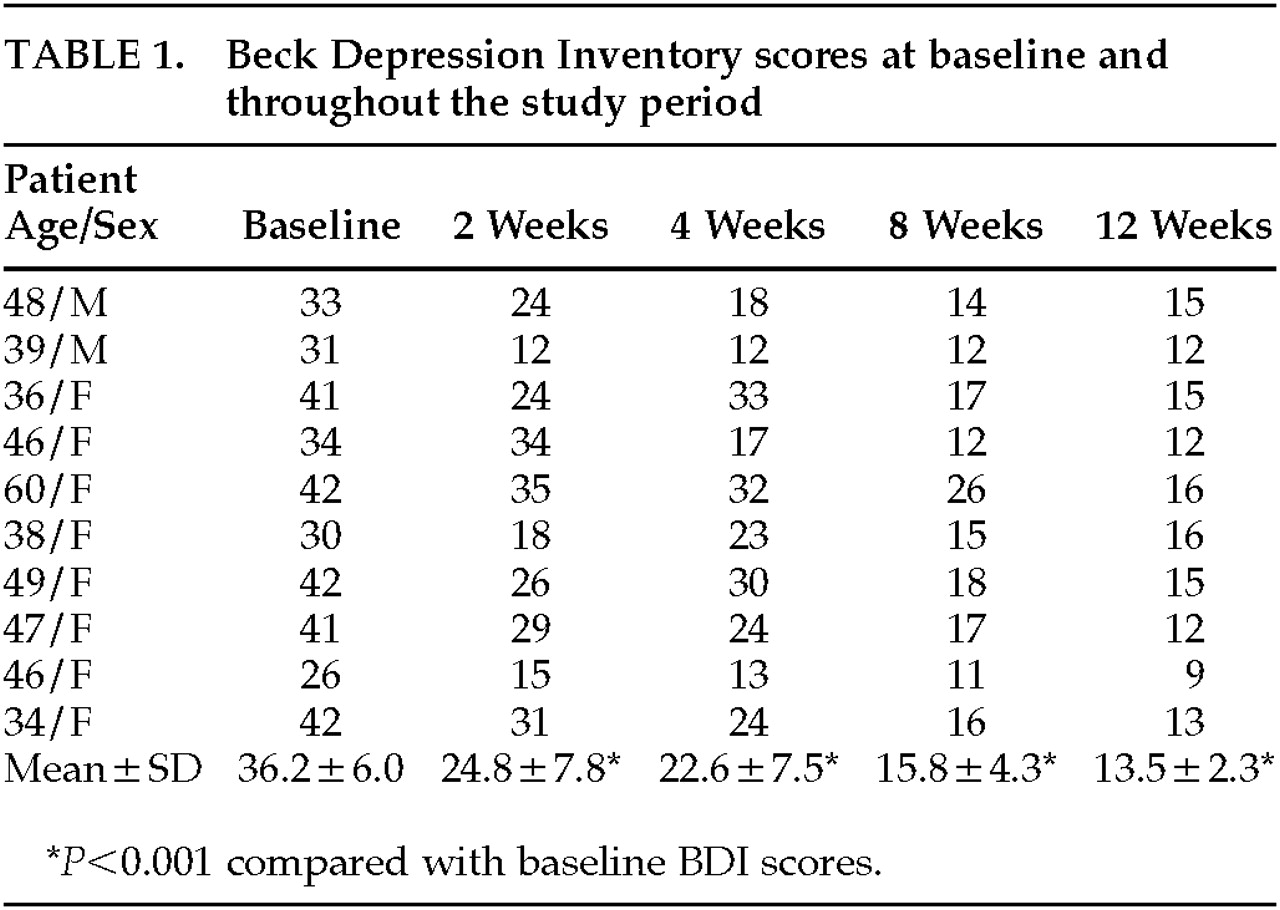
Periodic assessments, including vital signs (blood pressure, pulse), weight, physical and neurological examinations, and the Beck Depression Inventory (BDI),
10 were performed at diagnosis and at follow-up visits at weeks 2, 4, 8, and 12. The BDI was used to score severity of depression. The BDI includes 21 items of graded intensity and has high specificity and sensitivity for determination of levels of depression and assessment of the impact of drug interventions when a cutoff score of 17 is used.
11Monitoring of adverse events was carried out for each subject at each follow-up visit. An assessment was made of the seriousness, intensity, and relationship of any such events to the administration of the trial medication. An adverse event was defined as any unfavorable and unintended sign, symptom, or disease temporally associated with the use of moclobemide, whether or not related to this product. Documentation of adverse events was made by using the adverse event form in the patient's case report form and was based on the “Guideline for Good Clinical Practice” recommended by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
12Study Design
This was an open-label 3-month pilot study. The study was approved by the Hospital Internal Review Board and the Israeli Ministry of Health Ethical Committee. All participants signed an informed consent agreement following extensive explanation of the study's aims.
Patients were treated with moclobemide 150 mg/day for the first week. The dose was then titrated up to 600 mg/day according to side effects reported.
Inclusion criteria were 1) definite diagnosis of MS,
13 2) depression according to DSM-IV, and 3) severity at diagnosis by BDI >17. Exclusion criteria were 1) acute MS relapse; 2) known hypersensitivity to moclobemide; 3) current drug treatment with selegiline, cimetidine, pethidine (meperidine), or clomipramine; 4) pregnancy and/or lactation; and 5) participation in other research projects.
RESULTS
Ten MS patients (8 females, 2 males) were enrolled in the study. The mean age (±SD) was 44.3±7.7 years (range 34–60). All patients had a relapsing-remitting disease course. Disease duration ranged from 1 to 26 years (mean=12.6±6.7 years). Mean neurological disability according to the Kurtzke Expanded Disability Status Scale
14 was 3.3±2.1. At the time of diagnosis of the current depressive episode, 5 patients were drug-free, 2 were treated with intravenous immunoglobulin, 2 with interferon beta-1b, and 1 with copolymer-1.
The mean daily dose of moclobemide was 214 mg, ranging between 150 and 450 mg.
Depression severity at baseline as rated by the BDI score was 36.2±6.0. In 4 patients, the current depressive episode was the first episode; 6 patients had suffered from a major depressive episode in the past.
The mean BDI score decreased significantly after 2 weeks of moclobemide treatment (
Table 1). Upon completion of the study (12 weeks), the mean BDI score was 13.5±2.3 (
P<0.001). One patient elected to discontinue treatment after 2 weeks because of subjectively perceived remission. For the purpose of statistical analysis, his BDI score at 2 weeks was carried forward as the last observation.
Side effects, reported by 4 patients, included nausea (3 patients) and initial insomnia (1 patient). All were judged to be of moderate severity and not serious or life-threatening. A relationship to moclobemide treatment was judged to be probable. Nausea was reported to occur in all 3 patients within the first week of treatment and resolved without drug discontinuation within 7 to 10 days. Insomnia was reported at day 12 of treatment and resolved gradually by day 28.
DISCUSSION
In the present study we have shown the efficacy and safety of moclobemide in the treatment of comorbid depression in MS patients. There are only two other studies reported in the literature that specifically evaluated the effect of antidepressant treatment in MS patients. Schiffer and Wineman
5 conducted a double-blind controlled study of desipramine in 28 MS patients. Although improvement was significant, the experience of serious side effects in 30% to 40% of patients limited the dosage to half in the patients treated. Scott et al.
15 conducted an open-label trial of sertraline in 11 patients with stable MS who were suffering from major depression. They reported that all patients showed significant reduction in depressive symptoms, without significant side effects.
Our group has recently reviewed the treatment of depression in MS patients, emphasizing that this is especially complicated because of the side effects of the tricyclics, which include urinary retention, constipation, exacerbation of fatigue (sedation), reduced mobility, and adverse effects on sexual function.
16 Thus, optimization of therapy becomes related to pharmacokinetic parameters. Tolerability of the drug of choice becomes even more important because of the need for a continuation phase of therapy, typically extending 6 to 12 months after complete resolution of the current episode.
In the present study, the side effects of moclobemide were mild and transient, resulting in high compliance and enabling adherence to protocol. Our results support the theoretical basis for further research into the use of the reversible inhibitors of monoamine oxidase isoenzyme A in depressed MS patients.