SIR: Buprenorphine is a low-efficacy, partial mu-opioid agonist that has long been under investigation as an alternative to methadone for treatment of drug dependence,
1 with encouraging clinical results.
1–3 The drug has been introduced into clinical practice in many countries, including Germany, in recent years. Its unique profile includes a ceiling on agonist activity that decreases toxicity and risk for overdose. Its slow dissociation from the mu-opioid receptor results in a long duration of action, which offers the possibility of alternate-day dosing schedules. A possible benefit from buprenorphine treatment may also be less impairment of psychomotor performance and driving ability than has been described in drug-dependent patients under methadone maintenance.
4,5 We report preliminary data of an experimental study on buprenorphine's effect on driving ability under steady-state conditions in drug-dependent patients, using a standardized test battery, ART-90, which was developed in cooperation with the Austrian road safety board and validated in large groups of both healthy control subjects and patients.
4–6 Five subtests measuring peripheral vision, split attention, sensorimotor function, reaction time, stress resistance, and the capacity to integrate information were used to measure psychomotor performance and driving ability. These were 1) the Peripheral Vision Test with tracking task (PVT) to examine capability of perception and processing of peripheral visual information; 2) the Tachistoscope Test (TT15) to measure the capability to perceive relevant information quickly out of typical traffic situations (slide series presented for 0.75 s each); 3) Attention Under Monotony (Q1) to measure the attention for simple, monotonous stimuli presented for several minutes; 4) Reactive Stress Tolerance Test (RST3) to measure stress resistance and capacity to integrate information; and 5) DR2 Simple Choice Reaction Test to measure concentration (methods described elsewhere
4–6).
We studied 13 drug-dependent but otherwise healthy patients in stable substitution treatment with buprenorphine (negative drug screening). Mean age was 33.3 years (SD=9.1), and mean dosage was 6.5 mg (SD=5.8). Two patients were HIV positive. There was no clinical evidence for cognitive dysfunction in any patient; intelligence measured by the German test MWT-B indicated an average IQ of 106.
We compared the buprenorphine patients with 28 patients under methadone maintenance who had been studied before.
4 Mean age of methadone patients was 38.4 years; mean methadone dosage was 68 mg. Five were HIV positive without any evidence for cognitive dysfunction.
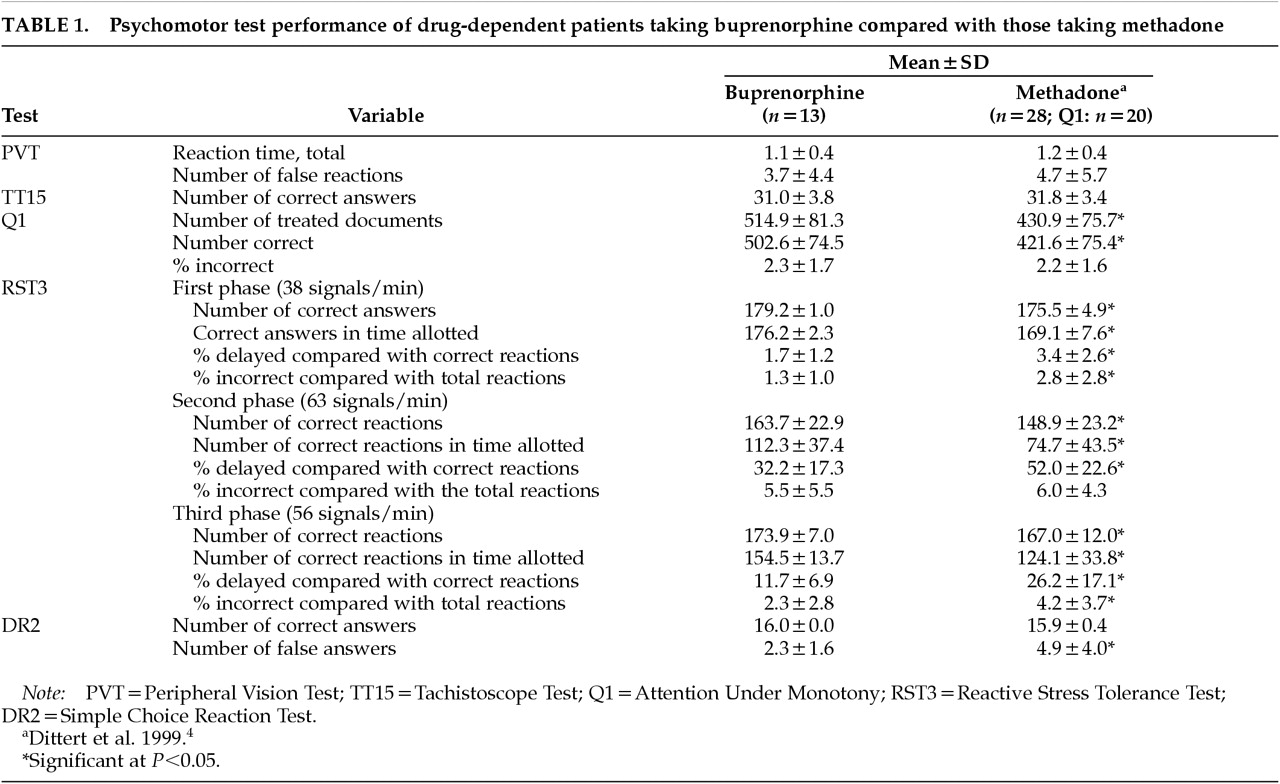
Results are shown in
Table 1. The data indicate an overall better psychomotor performance in patients under buprenorphine, especially in tests with stress components (RST3).
The clinical conclusions from this study are preliminary owing to the small number of patients tested and the a posteriori comparison with patients under methadone maintenance. Still, the comparatively better psychomotor performance of patients under buprenorphine compared with methadone maintenance, especially in the most sensitive tests for psychomotor performance under stress conditions (RST3) and monotony (Q1) is an interesting finding that deserves future attention. It may indicate a less marked effect on cognitive-motor performance of a mixed agonist/antagonist opioid than a full agonist such as methadone. A controlled clinical study to compare clinical effects of buprenorphine and methadone on psychomotor performance and driving ability in drug-dependent patients is planned to address this question.
ACKNOWLEDGMENTS
Supported by the Bund gegen Alkohol und Drogen im Strassenverkehr [Alliance Against Alcohol and Drugs in Driving] of Germany.