Ecstasy is the most common street name for 3,4-methylenedioxymethamphetamine (MDMA) or its analogues. It is also known as “xtc,” “adam,” “eve,” or “x.” It is a ring-substituted amphetamine that was first produced by a German pharmaceutical company, Merck, in 1912 as an appetite suppressant for German soldiers in World War I. However, it was quickly discarded because the empathic side effects observed on the battlefield were not considered desirable.
2 MDMA is classified as both a stimulant and a mild hallucinogen. Morgan
3 extensively reviewed the cognitive and emotional aspects of ecstasy exposure. Acutely, MDMA produces positive feelings including euphoria, increased self-confidence, increased sensory perceptions, empathy, intimacy, and openness. Negative acute effects include bruxism, tachycardia, hyperthermia, trismus (severe enough that users commonly resort to pacifiers or lollipops) and, more rarely, psychosis or death. The “crash” occurs 24 to 48 hours later, with muscle aches, depression, fatigue, and decreased concentration. The long-term effects are more controversial and probably relate to extent of use. Symptoms more consistently found in heavier users include depression, insomnia, anxiety, impulsivity, aggression, decreased learning and memory performance (recall and working memory), and, less frequently, decreased attention. Some or all of these symptoms may improve with prolonged abstinence.
3Doyon
4 recently reviewed the basic pharmacology and acute management of MDMA toxicity. Onset is in 30 minutes, with maximum effects in 1 to 3 hours and a half-life of 16 to 31 hours. Most commonly, ecstasy is ingested in the form of tablets or capsules, but it can also be smoked, injected, or absorbed as a suppository. The street drug is made in basements or garages, often with many additives to intensify the effect, including dextromethorphan (most frequently), caffeine, ephedrine, pseudoephedrine, and salicylates.
5 One interesting analysis of “ecstasy pills” revealed that 29% of tested samples contained no MDMA and 8% contained no psychoactive drugs.
5Many users consider ecstasy to be harmless. Medical literature on MDMA suggests otherwise. Mortality as a result of ecstasy is unusual, but it occurs.
9,10 Deaths have been attributed to hyperthermia, disseminated intravascular coagulation, fatal arrhythmias, acute myocardial infarctions, ischemic myocardial necrosis, and cerebral edema with cerebellar herniation and hepatic necrosis. MDMA may have a strongly nonlinear pharmokinetic profile. If so, a small increase in dose could lead to a substantial increase in plasma level and toxicity.
11A major challenge is the difficulty in designing studies that can clearly answer the question, “Is there anatomical injury from ecstasy use?” Inherent difficulties include unknown premorbid serotonin functioning; the effect of concomitant drug use (most users are polydrug users); reliability of self-reported usage; wide ranges in reported lifetime dose; variability in time from last dose; small group size; and difficulty recruiting a comparable control group. With these significant limitations in mind, a brief review and synthesis of existing imaging studies may aid the neuropsychiatrist in understanding the postulated effects of MDMA on the serotonin system, the potential secondary and tertiary effects mediated by vascular and other mechanisms, and possible implications for future health care demands. These reports on the effects of ecstasy demonstrate the progress being made in the study of brain functioning from an anatomical and neurochemical perspective.
MAGNETIC RESONANCE (MR) IMAGING AND MR SPECTROSCOPY
Most functional imaging studies include standard MR imaging, yet do not report any brain abnormalities in their ecstasy users. One study has reported a negative correlation between duration of ecstasy use and individual global brain volume (gBV), although there was no difference in mean gBV between the ecstasy and control groups.
13 Abnormal imaging findings (in globus pallidus and subcortical white matter) have been reported in ecstasy users who have survived initial toxic reactions.
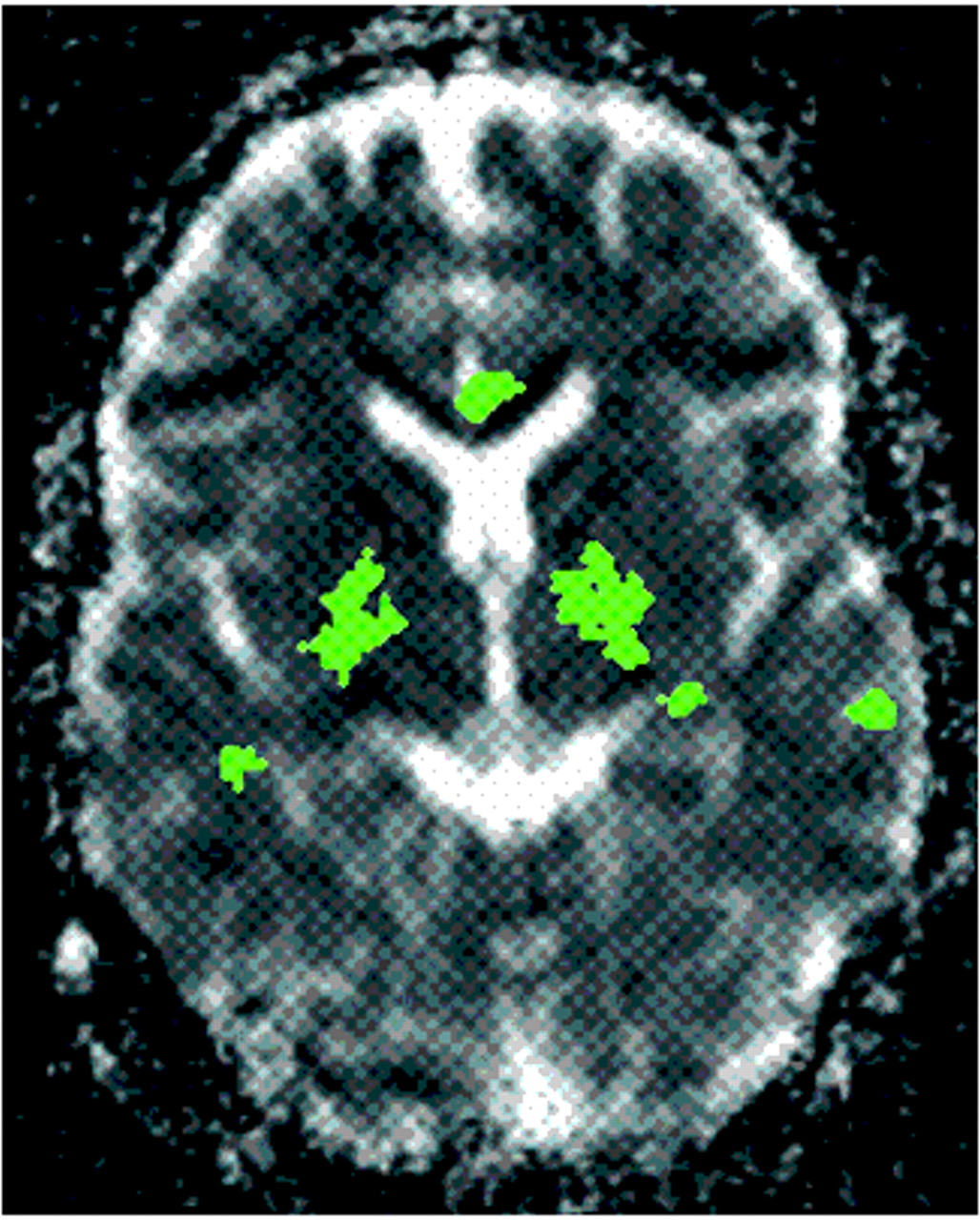
14–16It may be that lesions result from serotonin-induced vasoconstriction within the microcirculation, with the area affected often too small to be seen on standard imaging. Diffusion MR imaging measures the microscopic movement of water within tissue and is quite sensitive to microstructural changes. In a recent study, the apparent diffusion coefficient (ADC) was higher in some regions in 8 ecstasy users (abstinent for 3 or more weeks) compared with control subjects (
Figure 1).
1 In the same study, regional cerebral blood volume (rCBV) measured by contrast-enhanced MR imaging was also higher in these ecstasy users. The authors suggest that initially, ecstasy causes a release of serotonin and a decrease in blood volume due to serotonin-induced vasoconstriction. Serotonin is then depleted and a rebound vasodilation occurs.
Proton MR spectroscopy (
1H MRS) provides a way of measuring certain brain metabolites, presented as the spectrum of the amount of signal produced by the metabolites contained within a volume of brain. An MRS study of 21 ecstasy users (abstinent 0.5–26 months) found a significant increase in
myo-inositol (MI) content in parietal white matter of users compared with control subjects.
17 In addition, MI concentrations in both parietal white matter and occipital cortex were significantly higher in those users with the highest cumulative lifetime exposures. MI is thought to be a glial marker. Increases may reflect glial hypertrophy, perhaps an indication of brain insult or ongoing repair processes. Levels of
N-acetylaspartate (NAA, a neuronal marker), glutamate, and lactate were normal, suggesting that axonal degeneration has not occurred, at least not at a level measurable by this technique. In another study, the ratios of NAA to creatine and choline were lower in the frontal gray matter of users compared with nonusers, and reductions correlated with the extent of previous ecstasy use.
18 NAA levels in occipital gray matter and parietal white matter did not differ from the levels in control subjects. The discrepancy between these studies may, in part, be explained by higher ecstasy exposure in the second study.
FUNCTIONAL BRAIN IMAGING
Both single-photon emission computed tomography (SPECT) and positron emission tomography (PET) are nuclear medicine techniques that use radioactive tracers to image some aspect of brain function. SPECT has the advantage of wider availability; PET provides better image resolution, but is performed only in research settings. Relatively few functional imaging studies of ecstasy users have been published, and the available literature encompasses several very different measures. There is little in the way of replication of findings.
An important premise in functional imaging is that blood flow and metabolism rise and fall with brain activity. Thus, regional cerebral glucose uptake reflects regional cerebral metabolism and therefore neuronal activity. Several PET studies (using 2-[
18F]-fluoro-2-deoxy-
d-glucose; FDG) have looked for changes in regional brain metabolism related to MDMA exposure. A double-blind, placebo-controlled study examined the acute effects of MDMA in 14 drug-naive physicians and psychologists.
19 Subjects were evaluated with psychometric testing prior to and 90 minutes after drug or placebo intake, followed by FDG administration. During PET scanning, an auditory task was used to provide a more constant and reproducible mental state. Global glucose uptake was similar in the two groups. Regionally, the drug group had decreased glucose uptake bifrontally, and increased uptake bilaterally in the cerebellum and in the right putamen. Changes in cognition were correlated with decreased uptake in the frontal cortex, cingulate, and amygdala. The cortical results parallel known effects of other psychotropic substances and psychiatric illnesses. The authors note that these changes in the frontostriatal (thalamic) cerebellar network argue for a cerebellar role in cognitive and emotional processes.
Another group has used FDG PET to examine the relationship between total lifetime consumption of ecstasy, time since last dose, and regional cerebral glucose uptake in prefrontal cortex (Brodmann areas 10 and 11), striatum (caudate and putamen), and limbic areas (cingulate cortex, amygdala, hippocampus).
20,21 Their preliminary study found decreased uptake in the limbic areas and increased uptake in both frontal cortex and striatum. Their later, larger study found decreased uptake in all areas except area 10, which was increased. They found no relationship between total lifetime dose and regional glucose uptake. A correlation between time from last dose and regional glucose uptake was found only for the cingulate cortex. Interestingly, uptake was more severely depressed in those who started ecstasy use prior to age 18.
Regional cerebral blood flow (rCBF) is also considered to be indicative of neuronal activity. A double-blind, placebo-controlled study examined the acute effects of MDMA on rCBF measured with H
215O PET in 16 drug-naive students and hospital staff in 2 sessions separated by at least 2 weeks.
22 During imaging, all subjects performed a visual task to provide a more constant and reproducible mental state. The MDMA group had decreased rCBF in the precentral and paracentral lobule, dorsal anterior and posterior cingulate, superior temporal gyrus, insula, and thalamus. Increased rCBF was found in the ventromedial prefrontal, ventral anterior cingulate, inferior temporal, and cerebellar cortices. These blood flow changes were concomitant with elevated mood and changes in sensory/somatic perceptions. A later study from the same group found no differences in rCBF in 16 regular users versus control subjects while performing a simple cognitive activation task.
23 However, the study did not control for time since last drug intake.
Another group has used SPECT to evaluate rCBF, using xenon-133 (
133Xe) and technetium-99m hexamethylpropyleneamine oxime ([
99mTc]HMPAO) in 21 abstinent (0.5–26 months) users and matched control subjects.
13 They found no significant differences in rCBF and 2.3% lower global CBF in the abstinent users. Neither global CBF nor rCBF correlated with duration, frequency, or recency of ecstasy use. Ten of these users were then given MDMA and rescanned (8 subjects at 2 weeks post use and 2 subjects 2 months post use). The subjects rescanned at 2 weeks had decreased rCBF in most brain regions, with the largest decreases bilaterally in caudate and superior parietal cortex and the right dorsolateral frontal cortex (areas rich in serotonergic neurons). The decreases were more noticeable in the subjects given higher doses of MDMA. The 2 subjects scanned at 2 months showed increased global CBF. The authors suggested that these results may be related to the acute/subacute vasoconstrictive effects of MDMA-induced serotonin release. With time, an adaptive or neuronal recovery process may occur.
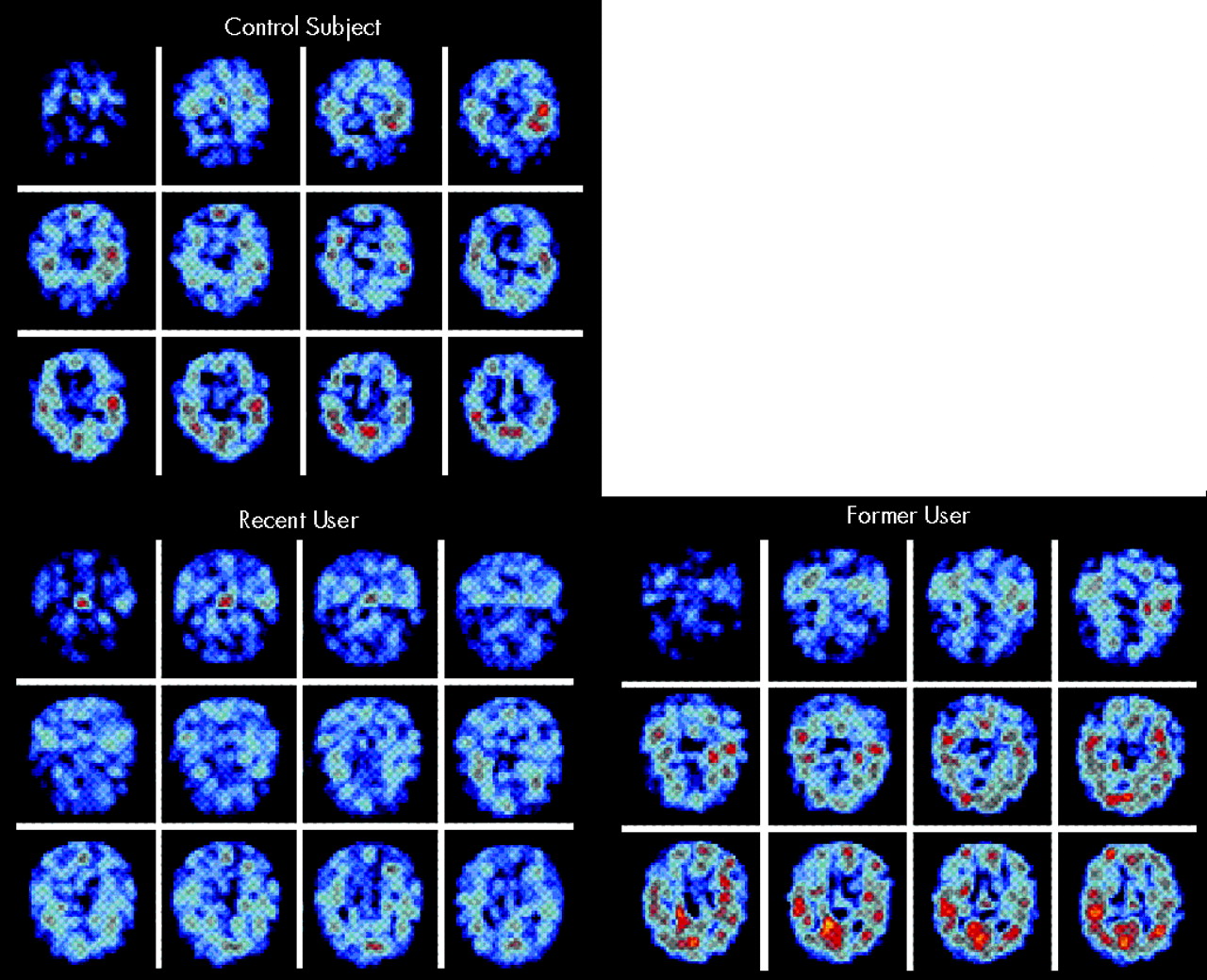
Another type of functional imaging directly evaluates neurotransmitter systems by measuring receptor binding. One group has imaged the postsynaptic serotonin receptor 5-HT
2A with SPECT (using [
123I]-5-I-R91150) in recent and former ecstasy users.
24,25 Binding was significantly decreased in frontal, parietal, and occipital cortex in 10 recent ecstasy users (abstinent 1–8 weeks, mean 7 weeks) as compared with 5 former ecstasy users (abstinent 8 or more weeks, mean 18 weeks) and 10 control subjects. The former users had slightly higher binding ratios than control subjects; this correlated with impaired performance on a delayed memory task (
Figure 2). In a companion rCBV study (using contrast-enhanced MRI) of a subset of each group, decreased rCBV and serotonin receptor binding were positively correlated in the globus pallidus and occipital cortex in 3 recent ecstasy users. Increased rCBV was found in 2 former ecstasy users. The authors suggest that ecstasy induces acute vasoconstriction via a large serotonin release that floods the postsynaptic receptors and triggers down-regulation; the resulting serotonin depletion causes a subacute up-regulation of receptors and vasodilation.
24 Both conditions could leave the user vulnerable to cerebral vascular accidents, especially in areas with a “watershed” blood supply (e.g., basal ganglia, subcortical white matter).
Both SPECT (using [
123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane, [
123I]β-CIT) and PET (using [
11C]McN-5652) have been used to image the serotonin presynaptic transporter (SERT).
26–29 In one study, 10 long-term ecstasy users were compared with control subjects matched for other recreational drug use. Ecstasy users had decreased binding in posterior cortical regions and a positive correlation between time from last dose and binding in the cingulate.
26,28 These investigators also found a decrease in cortical binding in current users (abstinent 3 weeks), but not in former users (abstinent 1 year or longer), compared with control subjects. Both the recent and former users had significant deficits in verbal memory, although these were not correlated with the SPECT findings.
The authors proposed several theories to account for these results, including the possibility of reversible injury; deficits not measurable by the current SPECT [
123I]β-CIT binding studies; abnormal reinnervation; or the deficits being postsynaptic in nature. Critics argue that SPECT [
123I]β-CIT binding is not reliable enough to make any firm conclusions and that concomitant marijuana use may have affected the results.
30In a related study, the same group found significant differences in the SPECT [
123I]β-CIT binding in female, but not male, ecstasy users as compared with female control subjects.
27 Decreased binding in females was dose-related and was evident even in the former users, but was not statistically different from levels seen in control subjects. The authors proposed that females are more susceptible to the effects of ecstasy and that these effects might be reversible. However, no sex-related difference was found in a PET study using [
11C]McN-5652.
29 SERT binding was decreased both globally and regionally (cingulate, frontal, occipital, and parietal cortices; striatum, cerebellum) in 14 ecstasy users (abstinent 3 weeks to >1 year) compared with control subjects. Binding correlated with extent of MDMA exposure, but not with time since last dose.
CONCLUSION
Although most users believe it to be harmless, most researchers agree that MDMA is to some extent toxic to human serotonergic (and perhaps dopaminergic) neurons. They also agree that some of the toxicity may be long-lasting. Imaging studies have examined structure, metabolites, blood flow, blood volume, glucose uptake, and serotonin receptor binding. Although results do not always agree, the most consistently implicated structures belong to the frontostriatal (thalamic) cerebellar network.
These studies are timely and crucial because some researchers are now advocating use of MDMA as an adjunct to treatment of posttraumatic stress disorder.
31 The findings may also have other public health implications. In particular, if permanent loss of serotonergic terminals occurs in some ecstasy users, this may pose clinically significant problems in later life as a result of decreased “serotonergic reserve.”
26 Finally, these neuroimaging techniques may give a way to further understand the complex interrelationships of the emotion and memory circuits and the role of serotonin in the human brain.