Posttraumatic stress disorder (PTSD) is a serious psychiatric illness with an estimated prevalence of 1% to 2% in the general U.S. population
1 and 15% to 25% in Vietnam combat veterans.
2 Studies have shown that PTSD in Vietnam combat veterans is associated with a wide range of social and psychological problems, including alcohol and drug abuse, suicidal ideation, depression, unemployment, marital and familial conflicts, suspiciousness, and a reduced social support system.
3,4 Current treatments for PTSD include medications, cognitive-behavioral techniques, and group therapy. Antidepressants have been the most intensively studied medications for treatment of PTSD, but their efficacy is variable and they are probably not as effective for combat PTSD as for noncombat PTSD.
5 For example, amitriptyline, a tricyclic antidepressant, was superior to placebo in reducing depression, anxiety, and PTSD symptoms in a controlled study of 46 veterans with combat PTSD.
6 However, a comparable controlled study with desipramine showed no difference between active drug and placebo.
7 Fluoxetine, a selective serotonin reuptake inhibitor, significantly reduced PTSD symptoms and improved depressed mood in patients with noncombat PTSD in a controlled study of 64 patients.
5 However, patients with combat PTSD improved only in mood, not in avoidance symptoms. There are comparable studies showing only moderate efficacy for paroxetine and fluvoxamine,
8–11 as well as for nefazodone,
12–14 bupropion,
15 and the monoamine oxidase inhibitor moclobemide.
16 Sertraline, another SSRI, is the only medication that is FDA-approved for treatment of PTSD on the basis of a placebo-controlled, multicenter study with 186 subjects.
17 This study demonstrated improvement in both mood and PTSD symptoms, but included very few combat PTSD subjects (6%). In an interesting “real world” study, Dow and Kline
18studied 72 combat PTSD patients in a real-world clinical setting but with standard PTSD and mood measures. They found that the probability of clinical response to each individual trial of antidepressant was only 20%, considerably less than in the controlled studies referred to above. These results reflect the experience of many clinicians that combat PTSD patients often have limited symptom response to antidepressant medications.
Repetitive transcranial magnetic stimulation (rTMS) is a new technique that has been found to be useful for investigations of cortical and cognitive function and more recently as a potential treatment for neuropsychiatric illnesses. There are several uncontrolled and controlled studies in which rTMS treatment led to mood improvement in major depression.
19–26 There are also two published open-label studies of rTMS in PTSD. McCann et al.
27 studied two patients with noncombat PTSD; the stressors were rape and a shooting incident. The patients' PTSD symptoms had been highly refractory to medications, and one patient had not responded to a trial of left frontal rTMS. Both patients had baseline cortical hypermetabolism on [
18F]fluorodeoxyglucose positron emission tomography (FDG-PET). They administered rTMS to right frontal cortex for 20 to 30 daily sessions at 1 Hz, 80% of motor threshold, administering 1,200 stimuli daily. After rTMS, PTSD symptoms decreased by about half and both global and right frontal cortical hypermetabolism decreased on FDG-PET. Grisaru et al.
28 administered rTMS to 10 patients with PTSD, with a stimulation rate of 0.3 Hz at the maximum output of a Magstim single-pulse stimulator, administering 30 pulses bilaterally over motor cortex with a nonfocal coil. They found transient improvement in both self- and observer ratings of PTSD symptoms. The transient nature of these improvements may be due to very conservative rTMS parameters, with only 60 repetitions daily as opposed to 500 to 1,000 repetitions typically in rTMS studies of major depression.
RESULTS
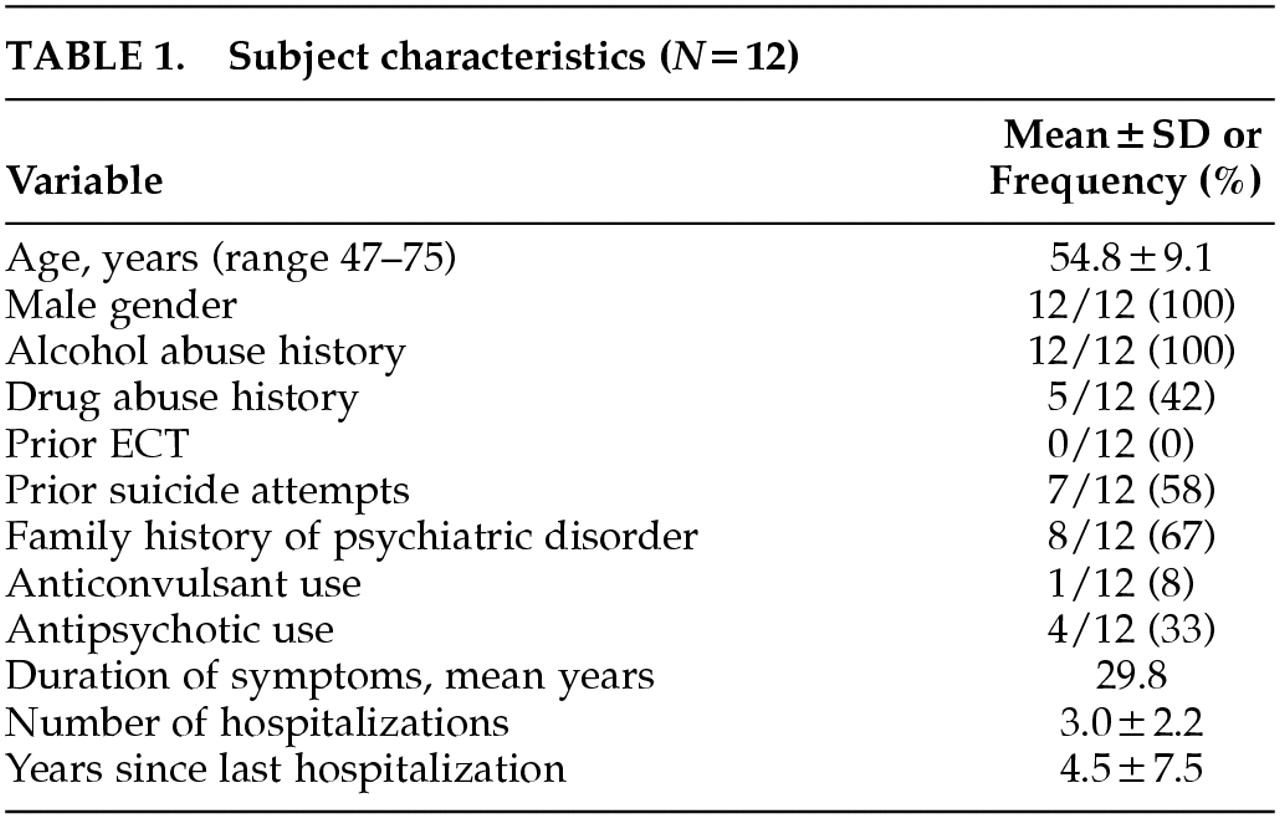
Fifteen outpatients gave signed informed consent and were enrolled in the protocol. Twelve patients completed the protocol. The characteristics of the study completers are outlined in
Table 1. All patients had a history of alcohol abuse and a substantial number had a history of drug abuse as well, which are typical findings in combat PTSD populations. No patients had a current diagnosis of substance abuse. There were no serious adverse events, seizures, or development of new neurological deficits. One of the 15 patients enrolled dropped out because of marked tension headache after two rTMS treatments; it is notable that this individual had an initial diagnosis of cluster headaches and rTMS was delayed for several months until he reached a quiescent point of his headache cycle; the headache symptoms he developed with rTMS were not typical of his cluster headaches but instead resembled tension headache. The other two patient dropouts never received rTMS: one patient did not meet inclusion criteria because of mood improvement prior to rTMS, and in the other patient we were unable to determine motor threshold at up to 80% of maximal output of the TMS machine, and higher stimulus intensities would not have been tolerated.
The patients were receiving a wide variety of antidepressant medications, including amitriptyline, fluoxetine, sertraline, paroxetine, venlafaxine, bupropion, nefazodone, trazodone, doxepin, and mirtazapine. Seven of the 12 completers were on two antidepressant medications and one was on three antidepressant medications.
Six patients received 1 Hz and six received 5 Hz rTMS. There were no statistically significant differences between the two groups in the demographic variables listed in
Table 1, nor in baseline clinical ratings (Ham-D, MISS, USC-REMT recall scores).
Clinical Efficacy
The results for the clinical measures are presented in
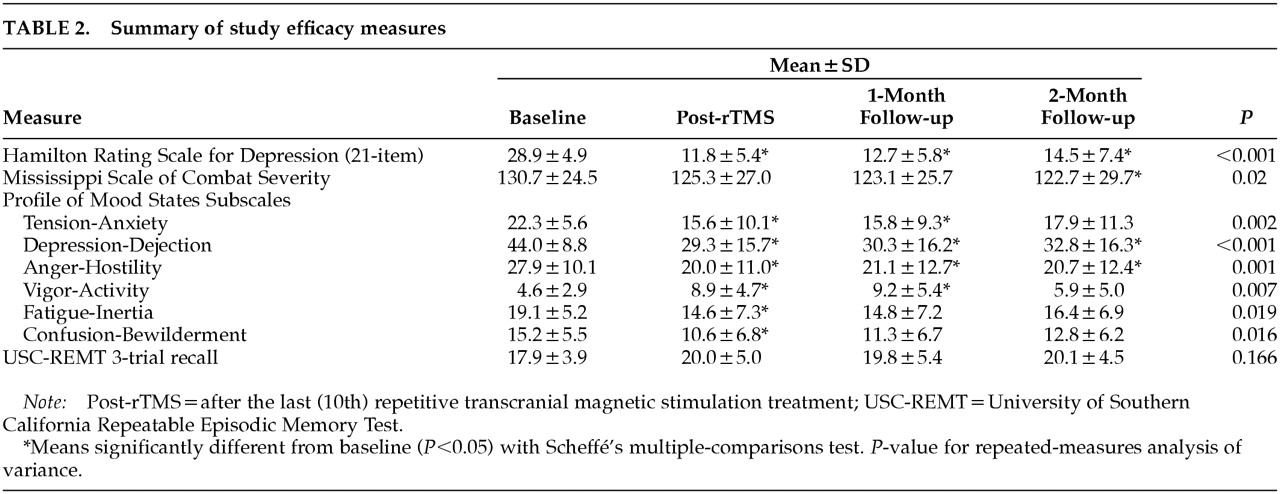
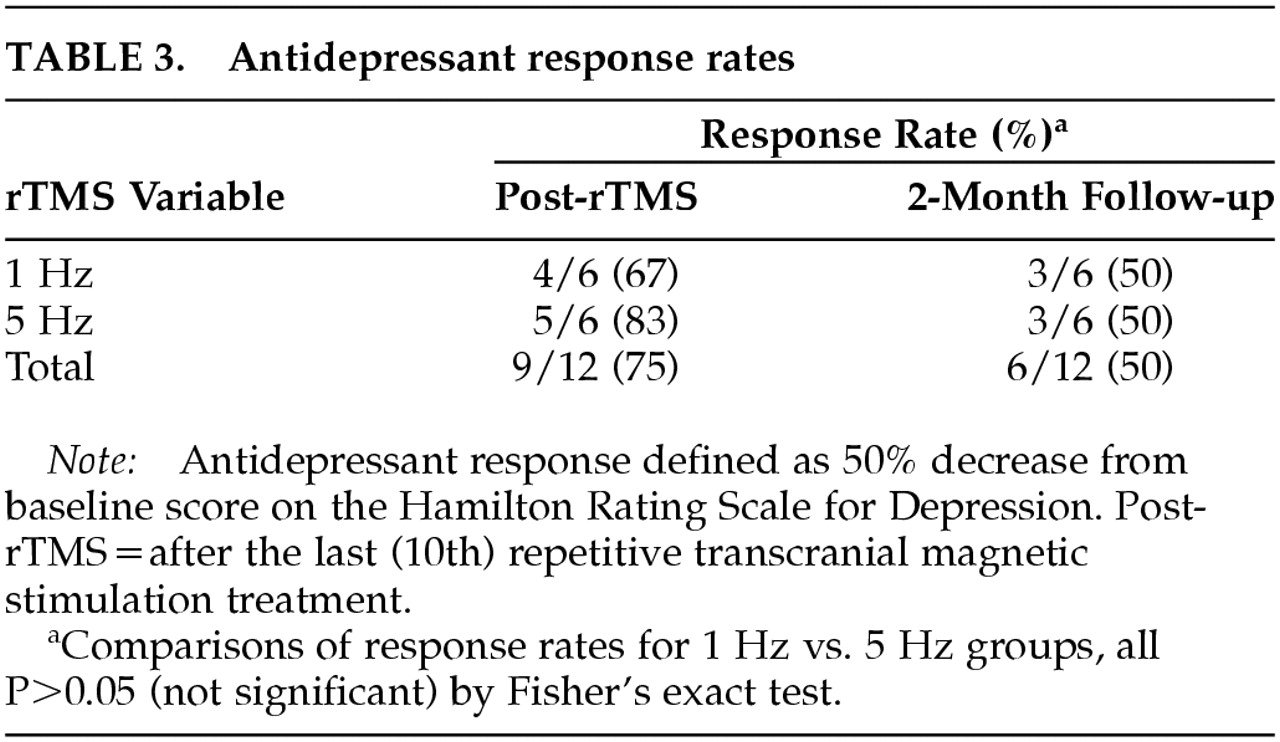
Table 2. Ham-D decreased robustly, and the decrease was largely sustained at 2-month follow-up. Rates of antidepressant response, displayed in
Table 3, show a 50% response rate at 2-month follow-up. Comparably sustained improvements were seen in the POMS subscales Tension-Anxiety, Depression-Dejection, and Anger-Hostility; the remaining POMS subscales improved transiently. Core combat PTSD symptoms as measured by the MISS showed a modest but statistically significant decrease (6%). Short-term recall as measured by the USC-REMT was unchanged after rTMS.
We found no significant differences in mood improvement between 1 Hz and 5 Hz rTMS (
Table 3), nor any differences between these groups in MISS or USC-REMT scores.
Subjective Sleep Changes
Patients anecdotally reported improved sleep after rTMS. Although we did not use a formal sleep assessment, we analyzed the three insomnia items from the Ham-D (early, middle, and late insomnia). The sum of these three items decreased from 5.0 at baseline (out of a possible 6.0) to 2.3 post-rTMS, 2.7 at 1-month follow-up, and 2.3 at 2-month follow-up (P<0.001 on repeated-measures ANOVA).
Time Course of Subjective Improvement
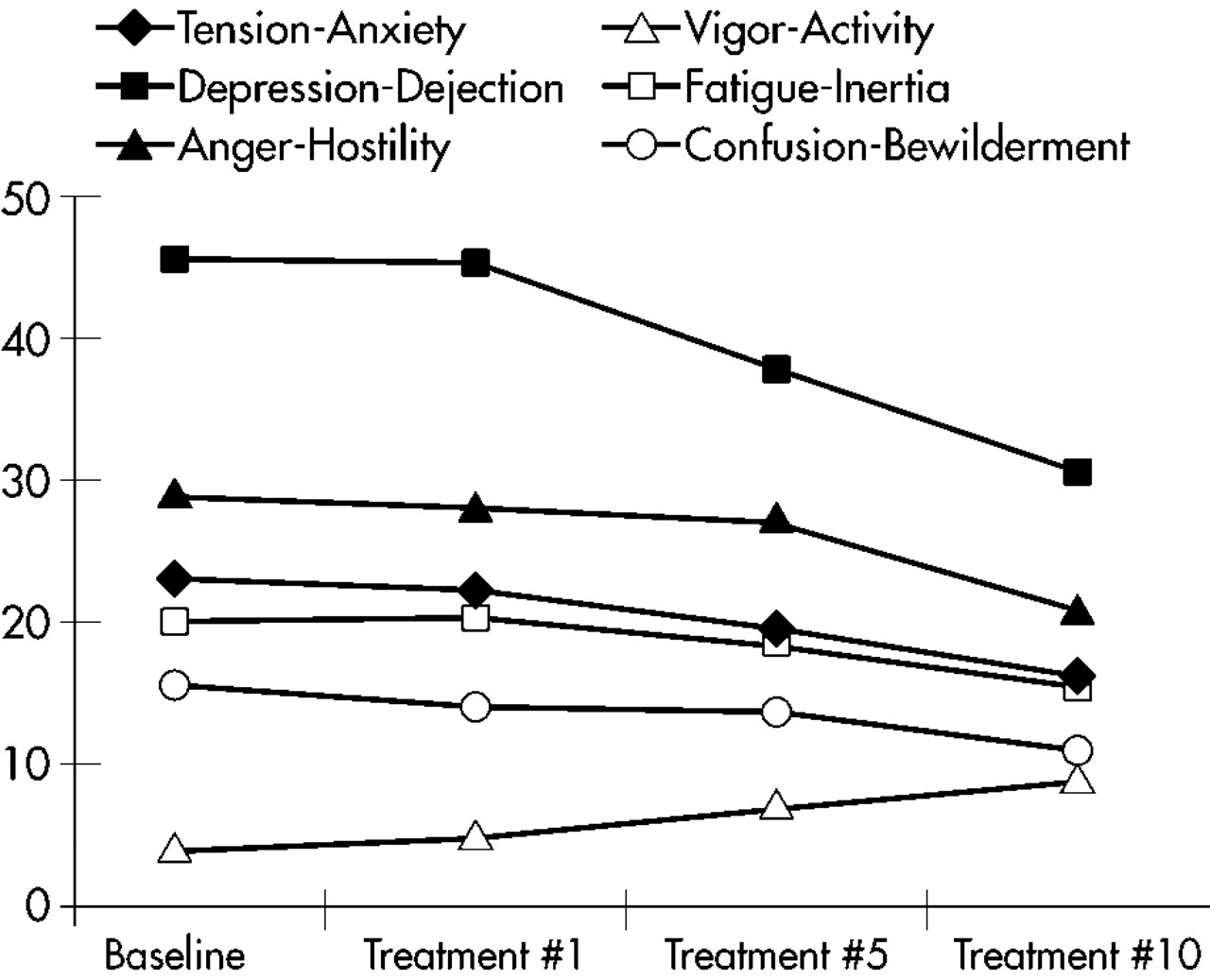
The rapidity of mood improvement was roughly linear through the 10 treatments. The POMS subscale scores during the course of the 10 treatments are graphed in
Figure 1. There is no apparent change after treatment #1. The POMS subscale scores after treatment #5 are lower but not statistically significantly different from baseline. The POMS subscale scores only become statistically significantly different from baseline at treatment #10.
DISCUSSION
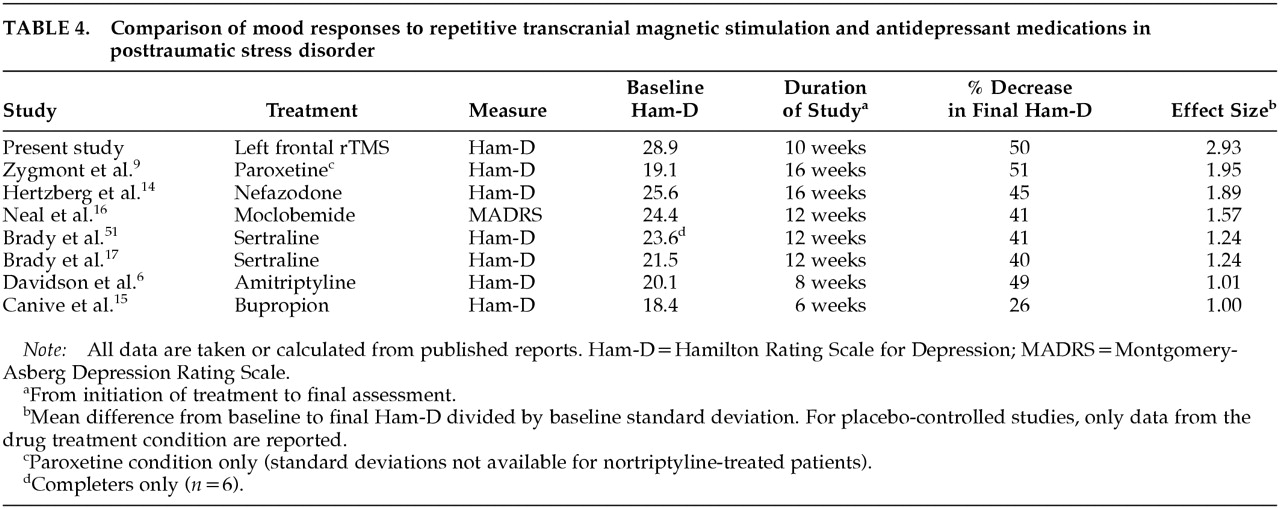
We found a clinically robust and sustained mood improvement after administering rTMS to patients with comorbid PTSD and major depression. Although we observed a slight relapse of depressive symptoms at 2-month follow-up, patients still remained substantially improved, showing a mean 50% reduction in Ham-D. The effect size and proportional reduction in depressive symptoms compare favorably with several controlled and uncontrolled studies of antidepressant medications in PTSD (
Table 4). Core combat PTSD symptoms improved only minimally, and this was consistent with patients' subjective comments that intrusive memories were not diminished by rTMS. However, the Tension-Anxiety and Anger-Hostility subscales of the POMS decreased, suggesting that affective distress did decrease even if intrusive memories, avoidance, and hypervigilance did not. Left frontal rTMS thus appeared to be effective against the depressive, anxiety, and anger symptoms that are common in PTSD, but not against the core trauma symptoms. Five treatments yielded only a partial and statistically insignificant response and 10 treatments appear to be needed for full response, in a finding similar to that of Klein et al.
26 Our patients' clinical profile is typical of VA PTSD clinic populations, with a high lifetime prevalence of substance abuse and chronic depressive symptoms.
Patients reported that their subjective experience of sleep was much improved. Because insomnia is one of the most common and distressing symptoms of PTSD,
37 improving sleep is crucial to PTSD treatment. Our patients reported 54% fewer sleep complaints on the sum of the three insomnia items on the Ham-D. This magnitude of improvement is similar to the 30% to 50% decrease in sleep complaints seen in antidepressant trials of PTSD.
8,9,12,14,16,38These data lead to the question of why we saw such a robust improvement in mood but only modest improvement in core trauma symptoms. We chose to stimulate left prefrontal cortex on the basis of prior reports of mood improvement in major depression. This choice of laterality is loosely based on observations that cerebral perfusion and metabolism are often decreased in left prefrontal cortex in major depression,
39,40 but comparable mood improvement has been observed after slow right prefrontal cortical rTMS.
26 Similar ambiguity exists for the treatment of PTSD; evoked trauma memories are observed to increase cerebral perfusion in right limbic areas,
41,42 which are not likely to be affected by left frontal stimulation.
Although our sample is too small to yield much statistical power, our preliminary finding that slow left frontal rTMS leads to mood improvements similar to those seen in fast left frontal rTMS is quite surprising. Fast rTMS (defined as ≥5 Hz) increases motor cortex excitability, and slow rTMS (1 Hz) decreases it.
43,44 Because left frontal cortical metabolism has often been found to be decreased in major depression, one might expect fast left frontal rTMS to “normalize” metabolism and thus be more effective than slow left frontal rTMS in improving mood, and the data of Speer et al. support this.
45 Our differing results may reflect the use of rTMS as an adjunct to antidepressant medications and the differences in patient populations.
The major limitation of the study is its open-label design, in which neither the patients nor the raters were blinded to treatment condition. There is considerable debate in the literature about the magnitude of placebo (sham) response to rTMS. George et al.
24 found an increase in Ham-D scores during the placebo phase of a crossover trial, suggesting no placebo effect. Similarly, Padberg et al.
46 found Ham-D scores slightly increased in their placebo group. Klein et al.
26 found a moderate placebo effect: a 23% decrease in Ham-D in their placebo group versus a 46% decrease in their active-rTMS group. Berman et al.
47 found no placebo effect, albeit the mood improvement they observed in the active rTMS group was modest. In contrast, Loo et al.
48 observed similar mood improvement in active and sham groups, but their sham condition may have caused significant cortical activation.
49 Given these questions about placebo effect in rTMS, any conclusions drawn from the results we have presented are necessarily preliminary. However, the magnitude of mood improvement we observed is considerably more than is usually seen in the placebo arm of rTMS studies or antidepressant medication studies,
17,50 suggesting that we have observed a true clinical effect.
In short, we observed substantial improvement in mood, anxiety, and sleep symptoms after adjunctive left frontal cortical rTMS in patients with comorbid PTSD and major depression. These encouraging results suggest that rTMS may be useful as an adjunctive treatment in this notably treatment-refractory population, and merit replication in a double-blind, sham-controlled design.