Poor performance on neuropsychological tests of problem-solving, memory, learning, perceptual abilities, and cognitive efficiency have been used to argue that chronic alcohol abuse results in brain dysfunction.
1,2 Other data indicate neuroanatomical changes due to alcohol abuse. Brain shrinkage in prefrontal and frontal areas that include both white and gray matter has been documented through autopsy and neuroimaging.
3–5 Significant callosal thinning has been noted in alcoholics ages 23 to 71 years, with and without nutritional deficient conditions, chronic liver disease, or dementia.
6,7 The amount of callosal thinning has been correlated with cognitive impairment involving visual/ logical memory and frontal lobe tasks, and with lifetime ethanol dose.
8,9 In addition, callosal agenesis has been noted in patients diagnosed with fetal alcohol syndrome (FAS).
10Hemispheric differences due to alcohol abuse have been difficult to document. Visual spatial processing, generally viewed as a right hemisphere skill, has been shown to be impaired in chronic alcoholics.
11,12 However, more specific damage from alcohol abuse that results in differences between hemispheres has been hard to assess, partly because conflicting results from studies of hemispheric functioning often stem from methodological differences.
13–17 Also, right and left hemisphere functions are so different that comparison of tasks used for assessment is very difficult.
18,19 However, emotional processing appears to be lateralized to the right hemisphere,
20 and a prior report from the study described here found severe deficits in affective prosodic comprehension (APC).
21 The present paper is focused on defining the neuroanatomical basis of the deficits found in APC among subjects with a history of alcohol abuse or fetal exposure.
Prosody is a nonlinguistic feature of language that is embedded into the articulatory aspects of speech through acoustic features such as pitch, intonation patterns, stress, timing, rhythm, and differential pausing. It is a suprasegmental feature that imparts information beyond that transmitted by articulation, word choice, and grammar.
22–25 Prosodic systems include affective prosody, which conveys emotions and attitudes; linguistic prosody, which connotes semantics and punctuation; dialectical prosody, which underlies regional variations; and idiosyncratic prosody, which reveals personal variations.
26,27 Linguistic prosody shares some of the acoustic features of affective prosody but is used for semantic purposes and to clarify potentially ambiguous syntax.
22 It appears to be incompletely lateralized to the left hemisphere.
28–32 Affective prosody gives the listener information about the speaker's emotional and attitudinal state, and allows the speaker to convey the same. Studies have shown that if the linguistic message is at odds with the affective prosodic content, the affective prosodic content usually takes precedence.
33–35 For example, if the sentence “I'm not mad!” is spoken in an angry tone, most people would believe the prosodic and attitudinal content rather than the linguistic content. Thus, the ability to modulate affective prosody is an essential element of face-to-face communication. The focus of this paper is on affective prosodic comprehension in alcohol-exposed individuals and its relationship to the neurologic and behavioral manifestations of affective prosodic impairments in brain-damaged patients.
Research suggests that modulation of affective prosody is a dominant function of the right hemisphere.
36–41 Right hemisphere lesions, depending on location, produce various aprosodias, which are deficits in comprehension and/or production of affective prosody. The right hemisphere's functional-anatomic correlates of affective prosodic comprehension (a posterior Sylvian function) and spontaneous production (an anterior superior Sylvian function) appear to mirror the left hemisphere's organization of propositional language.
36,38,42–44 However, the degree of lateralization of affective prosody has been questioned in light of reports from a number of researchers that aphasic patients with left hemisphere lesions may also have affective prosodic deficits.
32,45Because the presence of aphasic deficits may confound cognitive testing, the Aprosodia Battery
20 was developed to determine if affective prosodic deficits in patients with left brain damage (LBD) would improve under conditions of progressively reduced verbal-articulatory demands. This battery assesses both production and comprehension of affective prosody. In a study of patients with ischemic infarction, LBD patients showed a significant improvement in their ability to both comprehend and repeat affective prosody under reduced verbal-articulatory demands, whereas patients with right brain damage (RBD) showed no improvement in their performance.
20 Unexpectedly, the affective prosodic deficits in LBD patients were not associated with the presence or severity of aphasic deficits and had no relationship to the cortical distribution of the lesions. However, lesions involving the deep white matter adjacent to the corpus callosum seemed to better predict affective prosodic disturbances following LBD. This finding suggests that the predominant mechanism underlying affective prosodic deficits following LBD
20 is the loss of callosal integration of the dominantly lateralized language functions represented in each hemisphere (
LBD/Callosal profile), whereas the predominant mechanism underlying affective prosodic deficits following RBD
20 is the loss of affective communicative representations
39,40,47 and the ability to dominantly modulate affective prosody (
RBD/Aprosodic profile). Callosal integration is necessary to produce articulate speech with appropriate affective prosody that is temporally coherent.
20,48,49 The left hemisphere must apprise the right hemisphere of what words will be articulated and their rate in order for the proper application of stress and intonation to be delivered, and the right hemisphere must inform the left hemisphere of certain affective requirements in order to introduce emotional meaning into the propositional string.
Other pathological groups assessed with the Aprosodia Battery include schizophrenia patients, whose patterns of performance on the Aprosodia Battery were statistically indistinguishable from those of RBD patients.
50 Other findings for schizophrenia patients have shown that their reduced ability to process affect in face and voice is correlated with lower quality of life.
51 Apparently deficits in APC are related to poor psychosocial adjustment. Additionally, data from the normal elderly population show APC deficits related to age over 65 years, and their profile is unique when compared with LBD, RBD, schizophrenic, and younger control subjects.
Preliminary data suggest that a defect exists in affective prosodic comprehension among those exposed to high levels of alcohol.
21 Therefore, the goal of this study was to determine whether the alcohol-exposed pattern of performance on the affective prosodic comprehension portion of the Aprosodia Battery is similar to the RBD/ Aprosodia profile, the LBD/Callosal profile, a combination of the two, or an entirely unique profile. In order to answer these questions, we compared the pattern of deficits seen in detoxified alcoholics without prenatal alcohol exposure and in subjects with putative fetal alcohol exposure data collected on age-matched brain-lesioned patients who were tested previously on the Aprosodia Battery.
METHODS
Subjects
Subjects were recruited from two Veterans Affairs Medical Center facilities and were either patients or employees. Subjects agreed to participate in an approved study of emotional expression in speech and signed an informed consent form that was approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center in Oklahoma City. After screening for neurological injury or illness, subjects were scheduled for the 1.5-hour testing session. Participation was limited to individuals under age 65 years because deficits in APC have been discovered in the normal elderly population.
52 Subjects were 25 to 63 years old, were either African American or Caucasian, and were all right-handed. Detoxified alcoholics (ALC) with 21 days or more of sobriety who met criteria for a DSM-IV alcohol abuse diagnosis
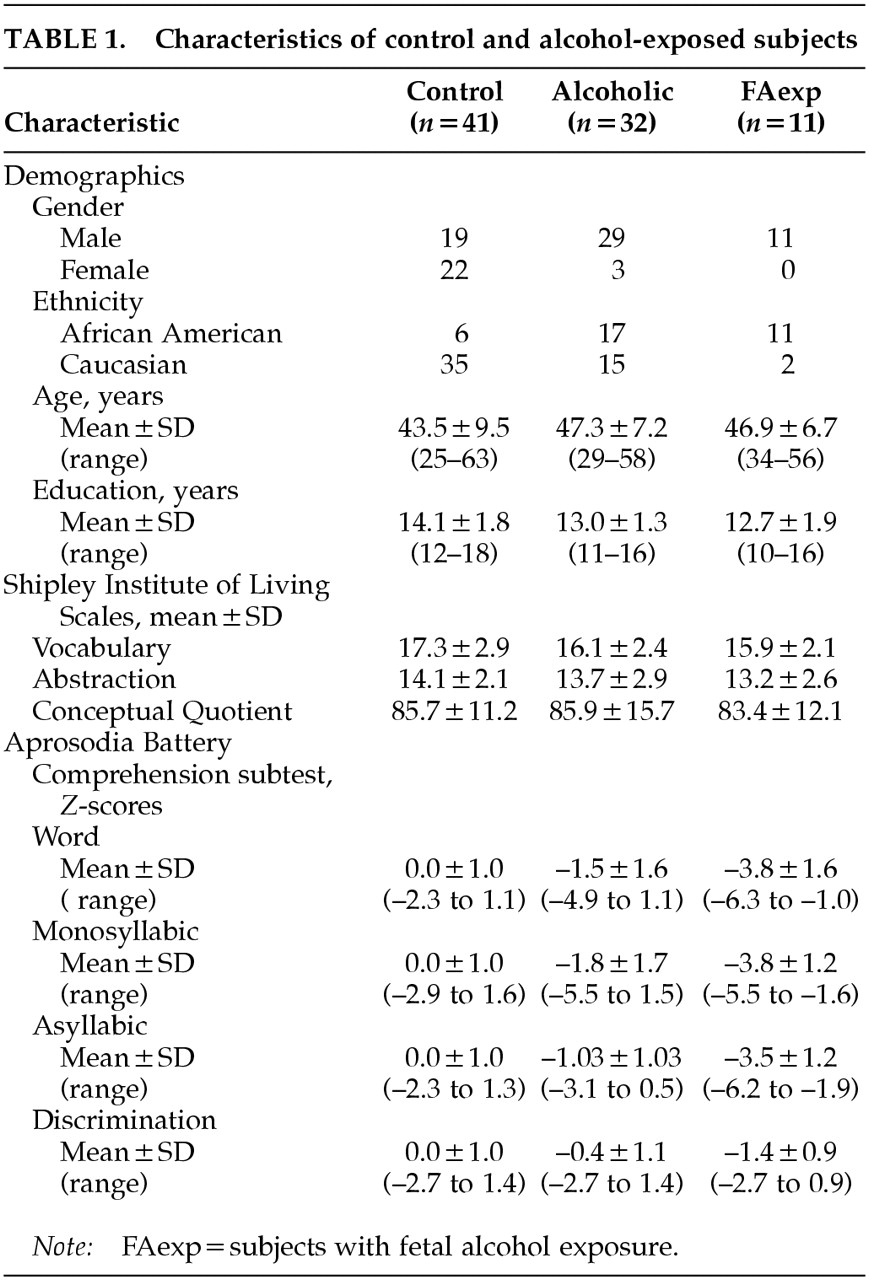
53 were scheduled for testing. Subjects who reported information indicating their mothers had abused alcohol during pregnancy were placed in a group of fetal alcohol exposure subjects (FAexp), whether or not they themselves had a history of alcohol abuse. The criteria for FAexp group placement included family history information such as mother's incarceration for public drunkenness and loss of custody of minor children due to alcohol abuse. Subjects with a Quantity Frequency Index of less than 5 ounces of absolute ethanol per drinking day were placed in the control group (CON) if they reported no history of alcoholism treatment or fetal alcohol exposure. Characteristics of control and alcohol-exposed subjects are shown in
Table 1. In order to provide comparisons with other populations, 10 left hemisphere brain-damaged patients (LBD) and 9 right hemisphere brain-damaged patients (RBD), age-matched to the ALC and FAexp groups, were included in statistical analyses of subtest performance patterns. These patients were selected from previously documented studies.
20,54Exclusion criteria for ALC and FAexp groups included serious neurological injury. In addition, all five subject groups were screened for neurological illness, chronic illnesses that result in significant restriction of oxygen to the brain, and DSM-IV psychiatric disorders other than substance abuse, and subjects were excluded if any of these were present. Medical records were examined for evidence of neurological injuries or serious illnesses; one alcoholic was removed from the final study group because of a closed head injury.
Test Materials
A health questionnaire was used during telephone screening for inclusion/exclusion criteria. The Shipley Institute of Living Scale
55 was used to screen subjects for cognitive impairment. The Alcohol and Substance Use Questionnaire, a structured interview instrument (copyrighted by the Oklahoma Center for Alcohol and Drug-Related Studies at the University of Oklahoma Health Sciences Center), determined extent of substance abuse in volunteers and their extended families. Thirty-two alcoholics (ALC) reported data indicating that their mothers did not abuse alcohol at any time during early child-bearing years. However, 11 subjects reported concrete behavioral data indicating that they were fetally exposed to alcohol (FAexp). Of these subjects, 9 were recovering alcoholics and the other 2 reported no lifetime alcohol use disorder. Forty-one control subjects (CON) were included in the final project group.
The Aprosodia Battery
20 assesses production, comprehension, and discrimination of affective prosody. The two major sections of the Aprosodia Battery are Production (spontaneous production and repetition of affective prosody) and Comprehension (identification and discrimination of conveyed emotion in speech). Subjects listen to compact disk recordings of three sets of utterances. The first Comprehension subtest, Word, uses a completely articulated sentence, “I am going to the other movies,” that is repeated using random variations of six emotions. The six emotions are Happy, Sad, Angry, Surprised, Disinterested or Bored, and Neutral. In addition, two stress patterns, with emphasis on “am” and with emphasis on “other,” test the subject's ability to suppress attention to linguistic prosody cues. Each exemplar is presented twice, resulting in 24 sentences in each subtest. Subjects indicate which one of six emotions they hear; exemplars can be repeated so that attentional variables are reduced. Twenty-four monosyllabic (“ba ba
ba ba ba“) and 24 asyllabic (“aaaaahhhhh”) utterances with the same emotional and stress patterns are also presented to the subject. The fourth subtest, Discrimination, presents 24 pairs of low-pass filtered exemplars from the Word subtest. The 12 affective-stress sentence combinations comprising the Word Comprehension stimulus set were played through a 70–300 Hz bandpass-filter and recorded. This procedure preserves prosodic-acoustic information involving intonation and intensity at both global (affective) and local (stress) levels while markedly reducing phonetic information. A stimulus compact disk was then made comprising 24 randomized sentences: 12 with the same affective intonation but different stress patterns and 12 with different intonations but the same stress pattern. For each of the 24 pairs, subjects must tell if the two sentences use the same or different emotional categories. Because these sentences employ different stress patterns, the task also tests the ability to discriminate between affective and linguistic prosody.
Statistical Analyses
The number of correct responses on each of the four Affective Comprehension subtests of the Aprosodia Battery (
Word,
Monosyllabic,
Asyllabic, and
Discrimination) were the primary dependent variables used in this study.
Z-scores were computed based on the CON group mean and standard deviation. Both SAS
56 and SPSS 8.0 for Windows (SPSS, Inc.) statistical programs were used to analyze data. For the variables reported in
Table 1, a mean was calculated for normal distributions, and a median to more accurately describe sample characteristics when outliers distorted the distribution. Variables were assessed by skewness figures
57 and the Shapiro-Wilk
W Normality Test
56 for their appropriateness in parametric analyses. Nonparametric tests used when variables did not show normal distribution were the Wilcoxon/Kruskal-Wallis tests (rank sums), median test (number of points above median), and Van der Waerden test (normal quantiles). Variance equality was assessed via the Bartlett; the conservative Welch analysis of variance (ANOVA) was used to test means when the variances were unequal. Post hoc analyses of estimated marginal means using Student-Newman-Keuls (homogeneous subsets and harmonic mean sample size with alpha=0.05) were used to ascertain significant differences on subtest scores for each group. Means comparisons were also assessed by using the Tukey-Kramer honestly significant difference test (significance level at <0.05) and the Student-Newman-Keuls post hoc analysis.
RESULTS
Group Demographics
Group characteristics are shown in
Table 1. Gender, ethnicity, and years of education were not associated with Aprosodia Battery scores among control subjects; one-way ANOVA and regression analyses performed on each of these variables with the four Comprehension subtests resulted in
P>0.10 on all tasks.
The scores earned by the three groups (CON, ALC, and FAexp) on the Shipley Institute of Living Scales (
Table 1) were analyzed by use of one-way ANOVAs. No significant differences were found between the groups on vocabulary, abstract reasoning, and a measure of cognitive impairment.
Aprosodia Battery Scores
The research goal was to determine if the profiles of scores from the four Aprosodia Battery Comprehension subtests for ALC and FAexp groups were similar to an LBD/Callosal profile, an RBD/Aprosodia profile, a combination of the two, or a unique profile. To explore this question, the patterns of deficits of ALC and FAexp subjects were compared with data previously collected on patients with unilateral ischemic strokes who were tested on the Aprosodia Battery at 4 to 6 weeks post infarction.
20,54 Because the research interest was on subjects with abnormal performance, the experimental groups were limited to individuals who scored <–1.64 SD (normal curve proportion <0.05) on at least one of the four Comprehension subtests. Group sizes, subtest means, and profiles are displayed in
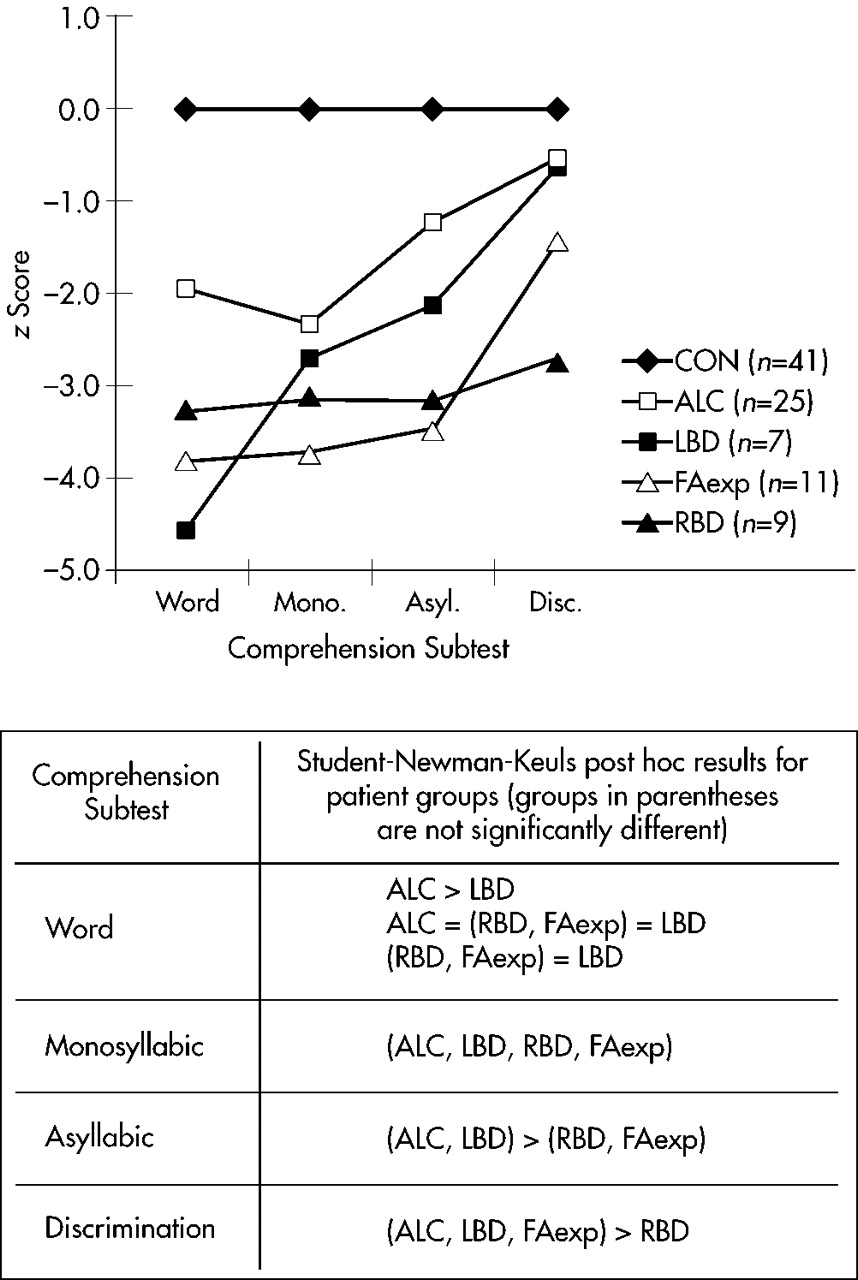
Figure 1. The data were subjected to a 5×4 repeated-measures ANOVA to compare means. A significant task by group interaction was observed (
F=5.60, df=12,264,
P=0.000), in addition to significant main effects for group (
F=53.98, df=4,88,
P=0.000) and task (
F=32.03, df=3,264,
P<0.000). Post hoc analyses using multiple 2×4 repeated-measures ANOVAs assessed how each patient group performed compared with CON. These tests showed highly significant task by group interaction (all
F>8.45, all
P<0.001), and main effects for group (all
F>61.62, all
P<0.001) and task (all
F>8.49, all
P<0.001) for the ALC, FAexp, and LBD groups. For RBD, however, no significant task by group interaction (
F=0.45, df=3,144,
P=0.71) or main effect for task (
F=0.46, df=3,144,
P=0.71) was observed, but a highly significant main effect for group was found (
F=99.89, df=1,48,
P=0.000). To assess how the four patient groups performed against each other, a 4×4 repeated-measures ANOVA was performed to compare means. It showed a significant task by group interaction (
F=2.54, df=9,144,
P<0.01) and highly significant main effects for both group (
F=10.58, df=3,48,
P=0.000) and task (
F=19.03, df=3,144,
P=0.000). A Student-Newman-Keuls post hoc analysis (alpha=0.05) was performed, with results shown at the bottom of
Figure 1.
Although the post hoc findings are somewhat complex, the following is evident by examining
Figure 1. The ALC profile (open squares) is not significantly different from the LBD profile (closed squares) for the Monosyllabic, Asyllabic, and Discrimination subtests, but shows a significant divergence on the Word subtest that suggests a partial RBD component. The FAexp profile (open triangles) is not significantly different from the RBD profile (closed triangles) for the Word, Monosyllabic, and Asyllabic subtests, but shows divergence on the Discrimination subtest that suggests a partial LBD component. Therefore, subtest performance on the Aprosodia Battery by ALC and FAexp could represent a combination of right and left brain-damaged profiles.
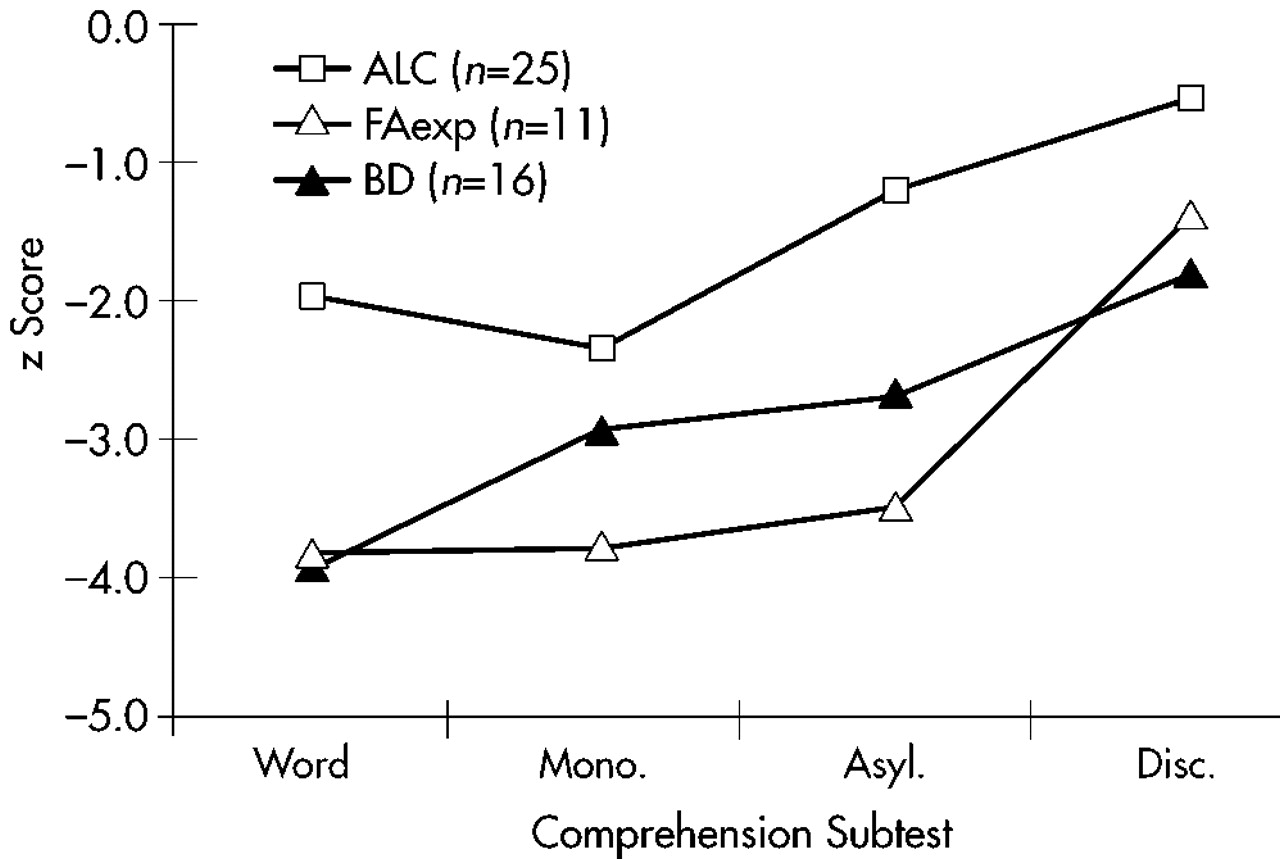
To determine if this supposition was correct, the LBD and RBD data were collapsed into a single brain-damaged group (BD). The subtest means and profiles are displayed in
Figure 2. A repeated-measures ANOVA was performed comparing BD, ALC, and FAexp group means. This time, the results showed no significant task by group interaction (
F=0.41, df=2,49,
P<0.67). However, significant main effects for group (
F=15.07, df=2,49,
P<0.000) and task (
F=42.61, df=2,49,
P<0.000) were still present. Since no interaction was observed, the Comprehension profile patterns of BD, ALC, and FAexp were statistically identical, supporting the supposition that alcohol-exposed subjects have a combination of LBD/Callosal and RBD/Aprosodic deficits.
The causes for the significant main effects were assessed by collapsing the data two ways—across groups preserving tasks and across tasks preserving groups—followed by separate one-way ANOVAs and Student-Newman-Keuls post hoc analyses (alpha=0.05) because no task by group interaction was present. The main effect for group was due to less severe overall deficits in ALC (mean Z-score=–1.52) compared with FAexp (mean Z-score=–3.12) and BD (mean Z-score=–2.82), which did not differ from each other. The main effect for task was due to the similar performance on the first two subtests, Word (mean Z-score=–2.94) and Monosyllabic (mean Z-score=–2.83), both significantly impaired compared with Asyllabic (mean Z-score=–2.15), which in turn was statistically worse than Discrimination (mean Z-score=–1.11).
DISCUSSION
A weakness of this study was the inability of the research team to confirm FAexp subjects' reports of mother's ingestion of alcohol during pregnancy; the mothers of all 11 FAexp subjects either had died, were suffering from dementia, or were unavailable to the subject at the time of the study. However, the criteria for inclusion were very conservative and have been discussed elswhere.
21 The small sample size for this group means that generalizations must be made guardedly until further studies have been completed, but because of the risk of missing important neurological impairments, this population should not be ignored in other research efforts. Another weakness of this study was that subjects were not tested for spatial-visual capabilities. Spatial-visual processing appears to be a dominant function involving the right posterior parietal cortex in strongly right-handed people and is usually found to be impaired in alcoholics. Another dominant right posterior cortical skill, however,
was tested, namely affective prosodic comprehension. Despite these weaknesses, the results are robust and the APC deficits are clinically significant.
The results of the statistical analyses from this study of affective prosodic comprehension in alcohol-exposed subjects may be summarized in two major points. First, the severity levels of APC deficits are quite different for the ALC group than for the FAexp group. This may indicate that ethanol exposure at different periods of human neural development produces different intensities of damage. Second, comparisons with scores achieved by patients with focal brain damage (BD) suggest that the patterns of deficits demonstrated by ALC and FAexp on the Aprosodia Battery are a combination of the RBD and LBD profiles: namely, dysfunction or damage involving the right cortex
and the corpus callosum. The LBD profile has been shown to be associated with deep white matter lesions adjacent to the callosum rather than to the distribution of left cortical lesions. The RBD profile is accounted for by right cortical lesion distribution.
46 Both the corpus callosum and the right cortex have been shown by imaging and neuropsychological testing to be atrophied and/or dysfunctional in chronic alcoholics and also in patients with FAS or FAE (fetal alcohol exposure).
All patient groups showed impaired comprehension compared with CON, but the overall severity of affective prosodic comprehension dysfunction varied (
Figure 1). Both FAexp and BD groups showed more severe deficits than ALC (
Figure 2). Although none of the alcohol-exposed subjects in this study had been diagnosed with FAS or FAE because they were born before 1973 when data were first published about the dangers of fetal exposure, the FAexp group was quite impaired on APC (
Table 1). Ordinarily, facial abnormalities, growth disorders, and pervasive cognitive dysfunction are typical criteria for FAS/FAE diagnoses. Apparently, some prenatal exposure to alcohol may be associated with the loss of specific cognitive abilities, even in the absence of symptoms meeting established FAS/FAE diagnostic criteria.
58,59 If the criteria for placement of subjects in the ALC and FAexp groups was an accurate reflection of when ethanol exposure occurred, the prenatal exposure resulted in the most damage to areas of the brain associated with APC.
A specific cognitive dysfunction due to ethanol exposure is suggested by these data. Exposure to alcohol had a deleterious effect on the affective-prosodic but not the verbal-linguistic aspects of communication. ALC and FAexp subjects were not different from CON subjects in the left hemisphere verbal skills subserving vocabulary and the frontal areas subserving abstract reasoning, based on screening with the Shipley Institute of Living Scales. In addition, none of the subjects had overt aphasic deficits. Each subject underwent an extensive clinical interview about substance use and family history by the first author, and no subject displayed speech or language difficulties requiring further formalized testing for aphasia or anomia.
Many clinicians and researchers assume that lexicon, grammar, and syntax are the most important factors underlying language competency, but emotional and attitudinal aspects are also essential. The detoxified alcoholics and the fetal alcohol–exposed subjects in this study performed similarly to focal brain-damaged patients with clearly established deficits in affective prosodic comprehension. Thus, one can assume communication competency was compromised. If this is so, it may help explain some of the social and employment problems experienced by alcohol-exposed individuals. Similar findings for schizophrenics have shown that their reduced ability to process affect in face and voice is correlated to severe psychotic symptoms and lower quality of life.
51 Social relationships, employment interviews, telephone conversations, and supervision sessions are some of the interpersonal situations that may be impaired if APC accuracy is significantly limited. These preliminary results indicate that early exposure to ethanol and/or excessive alcohol ingestion may have a deleterious effect on an important skill related to social interaction and communication, namely affective prosodic comprehension.
ACKNOWLEDGMENTS
This work was supported in part by a Merit Review Grant from the Medical Research Service of the Department of Veterans Affairs to Dr. Ross and a grant from the Tharp Foundation, Nashville, TN, to Dr. Monnot.