The most common neuropsychological deficits associated with traumatic brain injury (TBI) are alterations in memory and in executive and emotional functioning.
1,2 Typically these sequelae have been discussed in terms of selective damage to frontotemporal regions, including limbic structures, or similar indirect effects from phenomena such as transneuronal degeneration.
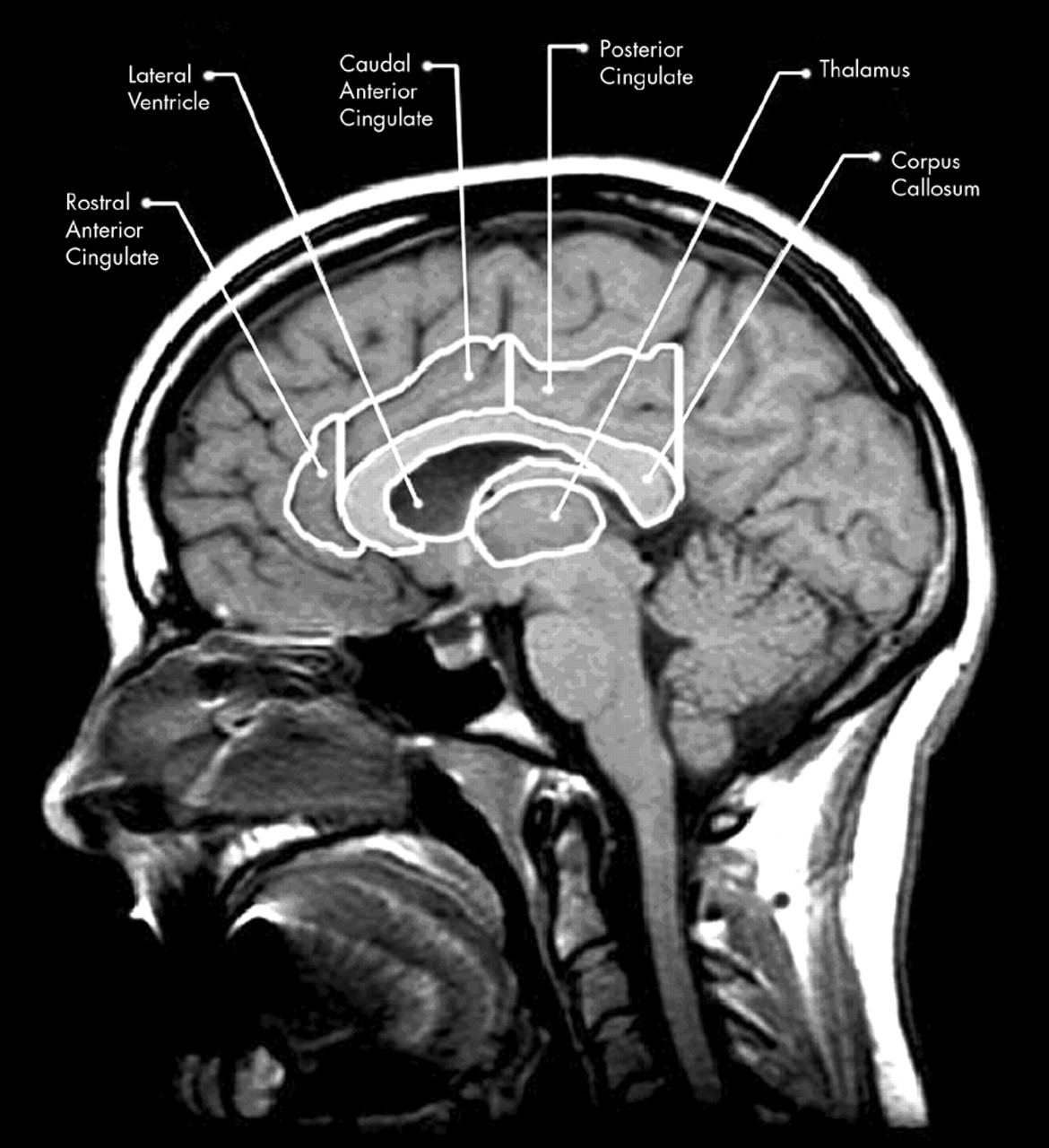
3–6 The largest and most recently evolved of the limbic structures, located medially in each hemisphere, is the cingulate gyrus (CG). The cingulate plays an important role in such diverse processes as integration of cognition and emotion, modulation of emotion-related autonomic activity, and emotional responses to pain.
7–9 Additionally, the cingulate has been shown to be involved in attention, executive processes, word generation, and memory.
10,11 Despite the functional significance of the cingulate gyrus in TBI, few studies have examined the anatomical and behavioral effects of acquired damage to this region.
A significant body of literature has suggested regional differences in the functional organization of the CG.
9 In particular, damage to the cingulate has been shown to affect a variety of neurobehavioral functions, producing affective disruption (anterior lesions) and memory and visuospatial dysfunction (anterior and posterior lesions).
7,9,12–16 Fontaine et al.,
17 using positron emission tomography (PET), reported significant relationships between reduced CG regional metabolic activity and neurobehavioral functioning in a group of 13 TBI subjects. In a functional magnetic resonance imaging (fMRI) study, McAllister et al.
18 reported that compared with more discrete bifrontal, biparietal, and cingulate activation in control subjects,
19 mild TBI subjects showed more widespread activation. Furthermore, McCrory and Berkovic
20 have asserted that in TBI subjects the “well-recognized behavioral and memory dysfunction is probably due to basal forebrain and limbic insults.” (p. 1491) It is noteworthy, however, that none of these investigations specifically examined cingulate morphology as a function of brain injury.
From a brain injury perspective, the cingulate may be protected because it lies deep in the cerebrum and the medial surface of each CG is supported by the falx cerebri during lateral movement. However, the position of the CG may be misleading, since it is vulnerable to herniation in cases of midline shift.
21 Moreover, damage to the cingulum following TBI may be caused by transneuronal degeneration rather than direct injury.
9 In light of these ambiguities, systematic investigation is needed to elucidate whether the cingulate shows structural changes in TBI and, if it is damaged, what the neurobehavioral sequelae are. Accordingly, in the present study we examined the relationships between cross-sectional CG surface area on magnetic resonance (MR) imaging and neurobehavioral status and injury severity in diffuse moderate to severe TBI.
RESULTS
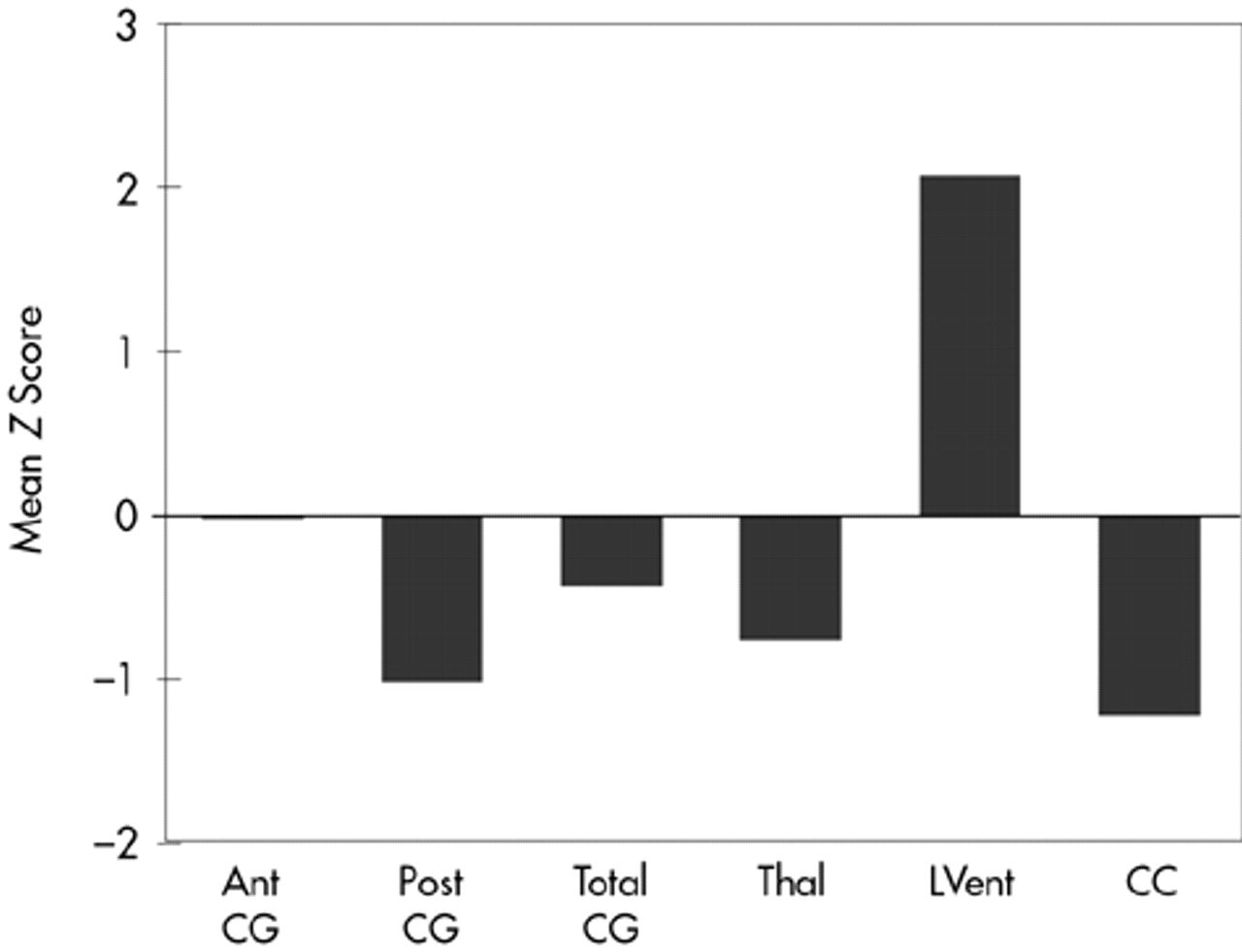
Results are summarized in
Figure 3. The MANOVA demonstrated several significant differences between control subjects and TBI subjects (
Figure 3). Total CG was smaller in TBI subjects compared with control subjects, although the difference was not statistically significant. However, significant atrophy was found in the posterior CG of TBI compared with control subjects (
F=6.17, df=1,27,
P=0.02). Decreased thalamic cross-sectional surface area was also found in the TBI subjects (
F=4.13, df=1,27,
P=0.05). As expected, the cross-sectional surface area of the CC was significantly reduced in TBI subjects (
F=6.52, df=1,27,
P=0.02). Similarly, lateral ventricular volume was significantly larger in TBI subjects compared with control subjects (
F=9.30, df=1,27,
P=0.004). TBI subjects were also significantly different from normal control subjects (results not shown) on whole brain volume (
F=18.15, df=1,27,
P< 0.001) and VBR (
F=10.79, df=1,27,
P=0.002).
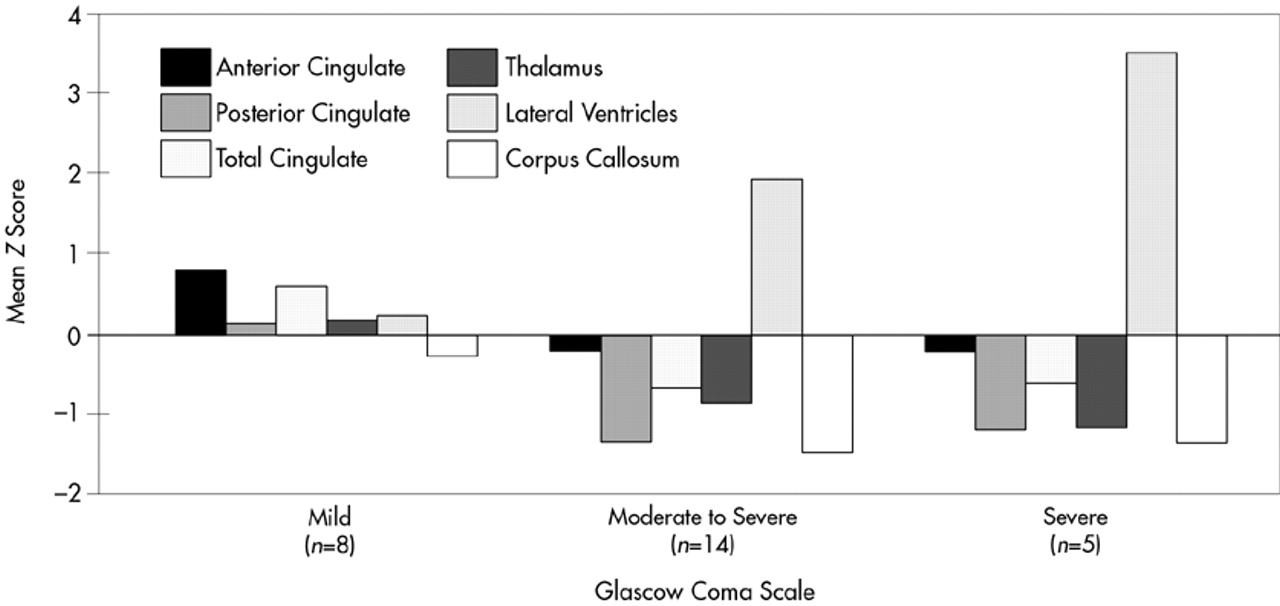
The TBI group was further divided into severe (GCS 3 to 6), moderate-to-severe (GCS 7 to 12), and mild (GCS 13 to 15) TBI, and the subgroups were compared for atrophic changes in brain structure (
Figure 4). In the severe and moderate TBI subgroups (combined) compared with the control group, significant atrophy was observed in posterior CG (
F=13.66, df=1,27,
P=0.001), thalamus (
F=7.93, df=1,27,
P=0.008), and corpus callosum (
F=10.67, df=1,27,
P=0.003), as well as significant enlargement of the lateral ventricles (
F=13.79, df=1,27,
P=0.001). For both the severe and moderate TBI subgroups compared with the mild TBI group, there was significant atrophy of the posterior CG (
F=5.16, df=1,27,
P=0.03), anterior CG (
F=5.88, df=1,27,
P=0.02), and thalamus (
F=4.87, df=1,27,
P=0.04), and significant enlargement of the lateral ventricles (
F=4.62, df=1,27,
P=0.04). In contrast, the mild TBI group showed much smaller and nonsignificant changes in brain morphology compared with the control group.
Greater impairment on neuropsychological test findings was associated with greater severity on GCS, but there were no significant correlative relationships between CG morphology and neuropsychological outcome. Similarly, the results of the Beck Depression and Anxiety Scales did not significantly correlate with CG morphology in the TBI subjects.
DISCUSSION
Traumatic brain injury has typically been conceptualized as an injury affecting diffuse neural systems that extend beyond the site of injury.
1–3,6,22,23,40 The assertion of diffuse injury is supported by the current findings, which show atrophic changes of the posterior CG, in the presence of generalized atrophy of the whole brain (i.e., increased VBR and decreased brain volume), CC, and thalamus, in TBI subjects. CG atrophy was associated with injury severity (GCS) but not with neuropsychological outcome. Several possible explanations for the lack of relationship between the CG atrophy and neuropsychological performance are discussed below.
First, it is important to note that atrophy of the posterior CG was primarily responsible for the overall reduction of CG surface area in TBI subjects. Unlike the CC, where front-to-back organization of fiber tracks corresponds with frontal-to-occipital anatomy, this organization is more distributed in the CG.
7,9,15,16,41,42 There are important frontotemporal connections with the CG that are organized posteriorly, including hippocampal input. Since the frontal and temporal areas are more likely to be damaged in trauma,
43 including hippocampal atrophy,
44 we would speculate that secondary neuronal cell loss from these regions may be a factor in the reduction of posterior CG surface area. In no case were lesions due to direct trauma or infarction of the CG, subjects with such lesions having been excluded from analysis. However, in the cases of severe TBI it is particularly plausible that direct mechanical deformation of the medial surface of the cingulate could strike the falx, causing injury. Accordingly, the significant posterior CG atrophy may have resulted from transneuronal degeneration secondary to frontotemporal damage, in the context of generalized, diffuse injury and resultant atrophy of the entire brain and/or direct mechanical contact with the falx.
Second, the lack of relationship between cingulate morphology and neuropsychological functioning may be due to the more indirect role that the CG plays in cognitive and emotional function and the fact that the cingulum is several synaptic connections away from the more direct cortical influence over behavior.
45,46 It is also possible that even though significant damage did occur in the cingulate, a threshold for impairment was not reached and compensatory recruitment of functionally related regions prevented direct neurobehavioral sequelae.
18 It may also be the case that more sensitive physiological measures such as PET imaging or fMRI are needed to show relationships between CG atrophy and behavior. Another factor is that left and right cingulate morphology were not separately examined because of sample size constraints. Lateralized cingulate function may relate more to neuropsychological function than an average measure of the cingulate. Also, we did not use traditional executive function tests, which often show impairment in TBI patients, so the relationship between traditionally measured executive functions and atrophy of the cingulate gyrus was not available for analysis. This is an area that should be examined in future research.
Third, the absence of anterior CG atrophy may be related to the greater anatomical variability of the more anterior aspect of the CG. As Ono et al.
30 demonstrated through detailed sulcal analyses, only 58% of their postmortem subjects had uninterrupted continuous cingulate sulci. This creates a potential boundary problem in precisely defining the cingulate, even with the anatomic atlases used in this study. For instance, one-half of the subjects in this study (3 with mild injury, 8 with moderate to severe injury, and 3 with severe injury) possessed “double parallel” or split gyri in the anterior cingulate that tended to have larger surface area than those with a “single” gyrus. Statistical comparisons of the double versus single anterior CG in predicting neuropsychological outcome were not significant. However, issues of sample size and statistical power may have affected the results of our analyses.
In a previous study, Connor
47 did not find a significant reduction in CG in TBI, but his measurement of the CG was taken in the coronal plane in a region analogous to the caudal anterior cingulate of the current study. In addition, he did not examine the single versus double phenotypes or the posterior cingulate. Nonetheless, this observation provides further support for the lack of atrophic changes in the anterior CG following TBI. It is apparent, however, that without a clear method for delineating anatomical variants, measurement error may obscure potential anterior CG–behavior relationships. We are not aware of any study that has systematically examined the neurobehavioral significance of single versus double-parallel cingulate gyrus morphology. Furthermore, the subjects with moderate to severe TBI in the present study did have significantly smaller anterior CG than the mild group, who actually had an anterior CG that was three-quarters of a standard deviation larger than control subjects (see
Figure 4). This finding further underscores the need to account for variability in CG morphology if CG–behavior relationships are to be understood.
The present study found posterior CG atrophy in TBI to be associated with severity of injury. Neuropsychological functioning was not significantly related to CG atrophy in the three regions examined, even when severity of injury was taken into consideration. Our study is limited in that we examined the CG in relation to structures commonly damaged in TBI, but we did not measure other limbic and frontotemporal neocortical structures such as the hippocampus and amygdala. These regions are vulnerable in TBI as well, and they are functionally related to the CG. It may be that other limbic structures need to be investigated to clarify relationships between CG atrophy and neurobehavioral outcome in TBI. Functional imaging studies of the CG have demonstrated relationships with executive and memory ability in TBI, so it is likely that CG atrophy does relate to the spectrum of dysfunction that accompanies TBI, although not through a simple linear anatomic connection. Indeed, even when the anterior cingulum is ablated, subjects may remain cognitively intact and experience a reduction of psychiatric symptoms.
48 In light of these observations, in several prospective studies, we are currently designing a line of research to elucidate the role of the cingulate gyrus in relation to functionally related systems in TBI and other disorders to more fully assess the relationships between CG morphology and neurobehavioral status.