Abnormalities within the thalamus and cortical-subcortical-thalamic circuits serving attention, sensory gating, and information processing have been suggested as a unitary explanation for the symptoms of schizophrenia.
1 However, both neuropathological and structural imaging studies have yielded heterogeneous results. Differences in methodology and possibly variability in patient characteristics contribute to the heterogeneous results and limit comparisons between studies.
Neuropathological studies examining the thalamus in schizophrenia are sparse, often involving small elderly populations with a long duration of illness who have received treatments (e.g., electroconvulsive and insulin therapy) with uncertain neuropathological consequences. In addition, these populations often have comorbid illnesses, and studies are further complicated by postmortem variables such as shrinkage. In studies using quantitative methodology, neuronal loss has been reported localized to the pulvinar.
2 Stereological studies have reported significant decreases in both dorsomedial nucleus (DMN) volume and DMN cell number, with preserved neuronal density.
3–5 Differential neuron loss in the parvocellular and densocellular subnuclei of the DMN has been reported.
6 However, others have reported normal total
7,8 and thalamic subnuclear
8 volumes. Decreased thalamic synaptic density
9–11 and decreased neuronal density in the anteroventral nuclei
12 have also been reported.
Structural imaging studies have also yielded inconsistent results, reporting both reduced
13 and normal
14 thalamic volume. Decreased pixel intensity in the lateral thalamus and adjacent white matter upon image averaging
1 was not replicated in a more recent study.
15 Shape analysis has suggested focal left anterior and right posterior thalamus changes.
16,17 Advanced structural magnetic resonance imaging (MRI), with slice resolution now approaching 1 mm, is likely to detect subtle atrophic changes that may have previously been unnoticed. However, subtle neuropathological changes not resulting in atrophy will remain undetected even with this advanced form of conventional MRI.
In vivo magnetic resonance spectroscopy (MRS) has also been used to study thalamic abnormalities in schizophrenia reporting both the presence
18 and absence
19,20 of reductions in
N-acetylaspartate, a marker that is thought to reflect neuronal loss and/or dysfunction. Thalamic hypometabolism has also been reported in functional imaging studies.
16,17Magnetization transfer imaging (MTI) is a novel structural MRI technique capable of detecting subtle neuropathological changes in vivo before atrophy becomes manifest and thus is of particular interest in the study of schizophrenia, where neuropathological changes tend to be subtle.
MTI in the brain is based on the interaction of protons bound to macromolecular structures (e.g., myelin and cell membranes), characterized by restricted motion, and free protons in tissue water, with unrestricted motion. Off-resonance irradiation is applied saturating the magnetization of bound protons, which are not detected by conventional MRI because of their very short relaxation times. There is an exchange of magnetization with free protons, resulting in a decrease in signal intensity that is dependent on the density of macromolecules in the brain. The exchange of magnetization between bound and free protons is measured by the magnetization transfer ratio (MTR). Reduced MTR reflects a reduced capacity of macromolecules to exchange magnetization with tissue water protons and is an index of the integrity of a given tissue.
MT has been used to study white matter diseases such as multiple sclerosis, detecting abnormalities in normal-appearing white
21 and gray
22 matter that are undetected by use of conventional MRI. Relatively small MTR decreases in the absence of myelin loss
23 are likely to reflect decreases in cell membrane protein secondary to neuronal loss, whereas very large decreases reflect myelin damage.
24 Neuropathological correlates of MTR in gray matter are not yet fully determined. In healthy volunteers, MTR is highly reproducible,
25 is higher in white than in gray matter (because of myelination), and decreases with age.
26 In our group's previous studies of chronic schizophrenic patients, we have shown the sensitivity of this technique to detect widespread cortical MTR reductions
27 and localized white matter pathology in the temporal lobes.
28 In the study described here, we measured MTR in the whole thalamus and two thalamic nuclei (DMN and pulvinar). We used a region of interest (ROI) methodology to identify subtle changes in these two nuclei. The selected nuclei have prefrontal, limbic, and temporal connections. These are considered to be important brain regions in schizophrenia
29 and are of sufficient size to accommodate the ROI, standardized at 35.2 mm
2. The DMN has connections with prefrontal cortex and amygdala, and the pulvinar has reciprocal connections with auditory association cortex.
30 Compared with other gray matter structures, the thalamus has a high MTR, reflecting the presence of myelinated fibers.
31 Decreased MTR in these ROIs is likely to reflect neuropathological changes in thalamic myelinated fibers and/or thalamic neuronal atrophy or loss. The null hypothesis is that there will be no differences in MTR between patients and control subjects.
METHODS
Subjects
Twenty-five patients (19 males, 6 females), mean age 37.2 years (range 25–46), who fulfilled DSM-IV criteria for schizophrenia were recruited from the Bethlem and Maudsley Hospitals. Patients were assessed by an experienced research psychiatrist (J.F.) using a clinical interview and careful review of patient records. Most patients (
n=23) were of the paranoid subtype. Mean symptom duration was 14.3 years (range 3–22). All patients were receiving neuroleptic medication. Five patients were being treated with atypical medication, but all had previously been treated with typical neuroleptics. Mean dosage (chlorpromazine equivalents; British National Formulary, 2000) was 367.5 mg/day (range 37.5–700 mg/day). No patients had been exposed to electroconvulsive treatment. Twenty-five healthy control subjects (19 males, 6 females), mean age 35.2 years (range 24–49), were also selected. Subjects with a history of neurological or systemic illness, head injury, drug abuse, or alcohol dependence were excluded. Schizophrenic patients and control subjects were matched for age, gender, and parental social class.
32 The study received approval from the ethics committees of the Bethlem and Maudsley NHS Trust, Institute of Neurology, and National Hospital for Neurology and Neurosurgery, London. Written consent was obtained from all subjects. These subjects had participated in our earlier studies.
27,28Handedness was assessed by using the Annett Questionnaire.
33 Most subjects were right-handed (schizophrenic,
n=24; control,
n=24). Premorbid IQ was assessed by using the National Adult Reading Test (NART)
34 and was available for 19 patients (mean score 110.7) and 19 control subjects (mean score 113.2). Positive and negative subscales of the Positive and Negative Syndrome Scale (PANSS)
35 were used to assess symptom severity during the week prior to scanning in schizophrenic patients. Most patients scored higher on the negative than the positive subscale of the PANSS, reflecting a preponderance of negative symptoms (mean total scores of 18.6 and 11.6, respectively).
Imaging
A GE Signa 1.5-tesla MR scanner with standard quadrature head coil was used. T
2-weighted and proton density images were acquired, using a dual echo sequence (echo time [TE]=15/90 ms, repetition time [TR]=3,000 ms, 28 contiguous 5-mm axial slices, 256×256 pixel image matrix, 24×24 cm
2 field of view). A spin-echo sequence (TE=30/80ms, TR=1,720 ms, 28 contiguous 5-mm axial slices, 256×128 pixel image matrix, 24×24 cm
2 field of view) both with and without an MT saturation pulse (16 ms, 23.2 microtesla Hamming appodized 3-lobe sinc pulse, applied 1 kHz from water resonance) was used for MT. The MT images were co-registered with the T
2-weighted and proton density images.
36,37 MTR was calculated on a pixel-by-pixel basis from the formula MTR=[Mo−Ms] / [Mo]×100, where Ms and Mo are the mean signal intensities with and without the saturation pulse, respectively.
Image Analysis
Data were processed on a Sun Ultra workstation (Sun Microsystems, Mountain View, CA), using the DispImage software package.
38 Intracranial volume included cerebrum, cerebellum, ventricles, and cerebrospinal fluid within the sulci and was determined by using a semi-automated technique. The cumulative total area of each 5-mm axial slice was multiplied by slice thickness to determine volume.
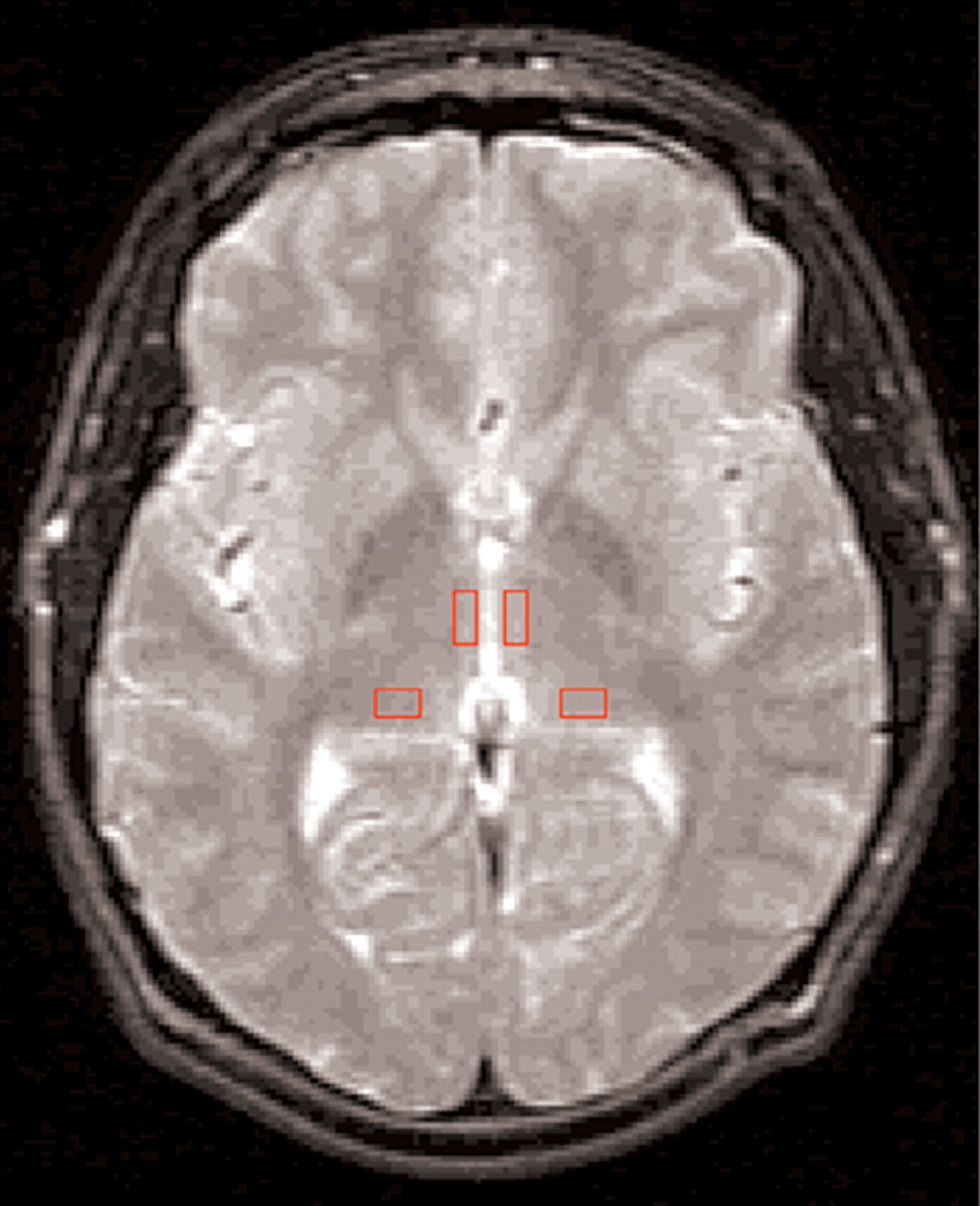
Thalamic boundaries and ROI localization (
Figure 1) were determined by using protocols developed in conjunction with a neuroradiologist (G.d.B.) with reference to standard atlases.
39,40 The thalamic boundaries were as follows: anterolateral: head of caudate; anteromedial: third ventricle; lateral: internal capsule; posterior: choroidal fissure of the lateral ventricle; medial: third ventricle; superior: body of the lateral ventricle; inferior: last slice in which thalamus was visible superior to the first slice in which both red nucleus and substantia nigra were visible.
Thalamic volume was determined by manually tracing thalamic margins on all slices in which the thalamus was visible (usually 4 to 5 slices) and multiplying cumulative total area by slice thickness. Mean thalamic MTR was determined by averaging cumulative MTR values for all thalamic slices.
Thalamic subnuclear margins are difficult to visualize with MRI. Thus, relatively large, anatomically relevant subnuclei were selected that could accommodate the ROI. Bilateral rectangular 35.2-mm
2 ROIs were manually placed in 2 consecutive slices containing the largest thalamic area (immediately superior to the anterior commissure) strictly according to the protocol (
Figure 1) by a rater (M.S.B.) who was blind to group membership. The ROIs were placed on the T
2-weighted image to avoid bias in placement, after adjacent slices were examined to minimize cerebrospinal fluid partial volume effects. The MT images were co-registered with T
2-weighted images,
36,37 allowing ROIs to be automatically transferred onto the MT images. The accuracy of ROI placement was qualitatively assessed by a neuroradiologist (G.d.B.) on random sampling of 6 controls and 6 patients. Mean MTR was determined for DMN and pulvinar by averaging MTR in both slices for each nucleus.
Interrater reliability for MTR ROIs was assessed for MTR values obtained in a random sample of 6 control subjects and 6 patients by 2 raters (M.S.B. and J.F.). Intraclass correlation coefficients ranged from 0.90 to 0.93 for all ROIs.
RESULTS
There were no significant differences in age, gender, handedness, or parental social class between patients and control subjects.
Volume Analysis
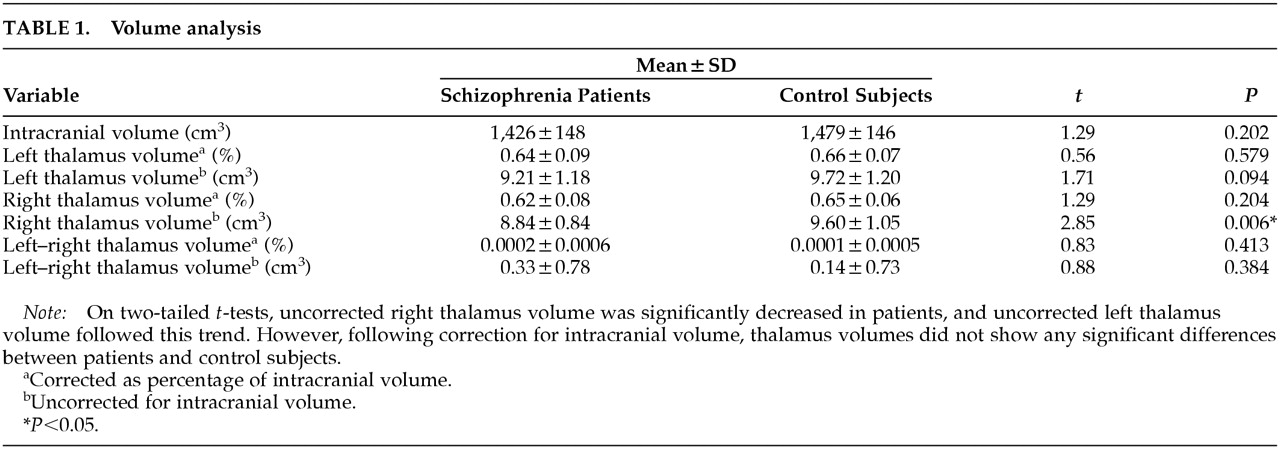
Two-tailed
t-tests revealed no significant differences for intracranial volume or thalamic volume corrected for intracranial volume (
Table 1). Schizophrenic brains were 3.5% smaller on the basis of mean values, but this was not significant (
P=0.202). Right thalamus volume was reduced in patients (
P=0.006) on the basis of uncorrected volume analysis, and a similar trend was seen for left thalamus (
P=0.094), but this reduction was not preserved after correction for intracranial volume.
MTR Analysis
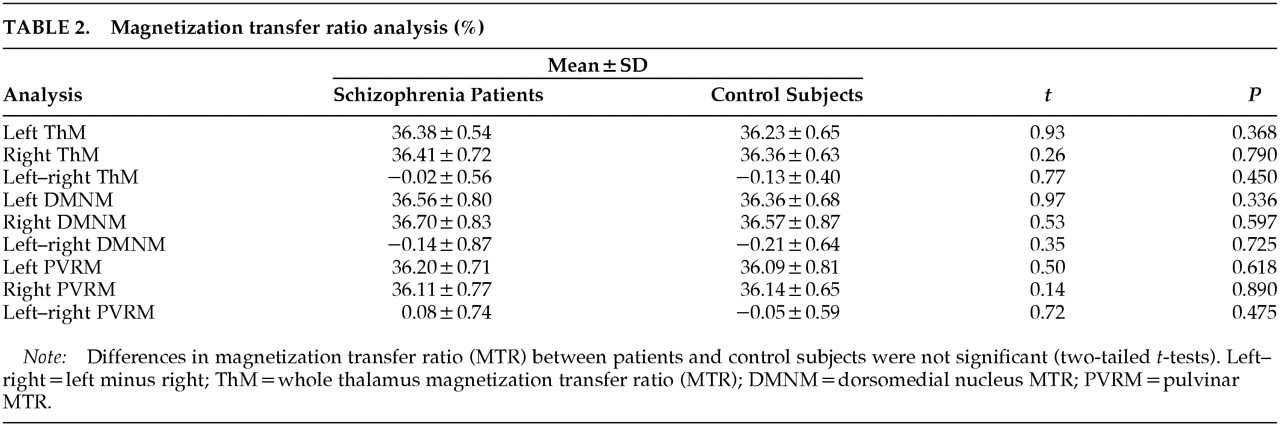
Two-tailed
t-tests did not reveal significant MTR differences between the two groups for whole thalamus, DMN, or pulvinar. Left minus right MTR values for the DMN and pulvinar were also similar, suggesting there were no differences in hemispheric asymmetry in the two groups (
Table 2). Using Spearman's correlation coefficient, we failed to find significant correlations of NART-estimated premorbid IQ or PANNS subscales of positive and negative symptoms with MTR in the pulvinar or DMN.
DISCUSSION
Using a novel MRI technique sensitive to the presence of subtle neuropathological abnormalities, we failed to detect thalamic volumetric or MTR reductions in patients with chronic schizophrenia compared with matched control subjects. In this study we focused on two thalamic nuclei with frontolimbic (DMN) and temporal lobe (pulvinar) connections putatively relevant in schizophrenia. The normal MTR values in the thalamus as a whole suggest that significant abnormalities in other individual thalamic nuclei not explored in this study are unlikely. Within-group comparisons of gender effects (6 females) or schizophrenia subtype were not possible because of the small sample size and the fact that most patients were of the paranoid subtype. Therefore it is not possible to say whether MTR abnormalities may have been present in other clinical subgroups. We also failed to find correlations between NART-estimated premorbid IQ or PANNS subscales for positive and negative symptoms and MTR in the pulvinar or DMN. However, because of our small sample size we cannot exclude the possibility of thalamic abnormalities in subgroups of patients with earlier-onset schizophrenia, as suggested by some studies,
41 or in those with specific symptom clusters or cognitive impairment.
The neuropathological correlates of MTR are not fully understood, but there is substantial evidence that MTR is sensitive to changes in tissue organization and macromolecular structure.
21–23 Medial temporal cortical
27 and temporal lobe white matter
28 MTR abnormalities have been demonstrated in the same patients, supporting our view that the technique is sufficiently sensitive to detect the subtle neuropathological abnormalities in schizophrenia.
Differences in thalamic volumes between our patients and control subjects did not survive correction for intracranial volume. Better slice resolution may have increased our chances of detecting volumetric differences between the two groups, although a high-resolution study using 1.5-mm contiguous slices in a sample of 15 right-handed patients with chronic schizophrenia also reported no differences in thalamic volumes between the patients and control subjects.
14 Others using lower-resolution image acquisition have reported significant thalamic volume reductions in schizophrenic patients,
13 and these conflicting results may, in part, be attributed to methodological differences including border definition, signal-to-noise ratio, and slice acquisition parameters. The use of high-resolution techniques (i.e., CAIR, or cortex-attenuated inversion recovery MRI sequence) that allow visualization of specific thalamic nuclei
42 may help to settle the differences between various studies.
The main limitations of our study are the small sample size, possible partial volume effects, and limited resolution dictated by 5-mm contiguous slices that may not be sensitive to subtle localized volume changes. A previous study by our group
28 reported a 2% difference in MTR between chronic schizophrenic patients and control subjects in the right temporal lobe, which was highly significant, and a retrospective analysis revealed that 16 patients and 16 control subjects would have been sufficient to detect differences with a power of 80% and a significance level of 0.05. In the present study, the maximum difference between patients and control subjects for MTR was in the left DMN (0.20%), and the standard deviation for control subjects was 0.68%. On the basis of these variables, if significant differences were present between patients and control subjects in the left DMN, we would require a sample of 400 subjects to detect a difference with a power of 80% and a significance level of 0.05. Larger samples would be required for the other nuclei tested in this study. Arguably, differences of this magnitude may not be biologically relevant.
A better understanding of the neuropathological correlates of MTR is required before our findings can be interpreted conclusively. However, with these caveats and the need for replication in mind, our results do not support the presence of gross pathological changes in the thalamus. If present, thalamic abnormalities are likely to be far more subtle than those present in the cortex in chronic schizophrenia that are detectable with the same technique. Our findings do not exclude state-dependent abnormalities in functional connectivity
43 but suggest that these are more likely to result from cortical than thalamic abnormalities.
ACKNOWLEDGMENTS
The authors are grateful to members of the NMR Unit for their assistance and also thank the patients and control subjects who participated. This study was supported by a grant from the Wellcome Trust.