Reproductive events such as menarche, pregnancy, and menopause influence a woman's risk for a number of diseases. For example, the incidence of breast cancer is higher in women who have longer estrogen exposure due to early menarche, late menopause, or nulliparity.
1,2 Conversely, a naturally high exposure to estrogen over a lifetime may decrease the chance of developing osteoporosis.
3 Some studies also indicate that estrogen influences the risk for age-related diseases of the central nervous system. A large body of basic science research has provided possible mechanisms by which estrogen might influence brain aging. These include modulation of acetylcholine
4 and other brain neurotransmitters, enhanced dendritic sprouting,
5 interaction with trophic factors,
6 prevention of cerebral ischemia,
7 protection against oxidative stress,
8 and modulation of lipoprotein isoforms.
9 Clinical studies of estrogen replacement therapies have shown mixed results, with the two published prospective studies demonstrating a reduced risk of Alzheimer's disease (AD) with hormone therapy
10,11 and retrospective studies yielding mixed results.
12–15 Current data do not support the use of hormone replacement therapy (HRT) in the treatment of AD.
16–19 Studies of HRT on age-related decline in particular cognitive domains have also produced mixed results,
20–23 with positive studies showing an appreciably consistent effect on verbal memory.
21,23–25Little is known about the effects of endogenous estrogens on cognition across the lifespan. A few studies have assessed the risk of developing Alzheimer's disease associated with reproductive events that affect lifetime endogenous estrogen exposure. One study showed a significantly increased risk of Alzheimer's disease associated with advanced age at menarche, but not advanced age at menopause.
26 Another found the opposite pattern of results,
13 and two others found no effect for either factor.
11,27 Studies of the effect of reproductive factors on cognition in nondemented postmenopausal women have involved small samples (i.e., around 100 women), and suggest that reproductive events associated with higher lifelong estrogen exposure may be associated with enhanced cognition. Two of the studies reported that early menopause is associated with poorer cognitive function,
28,29 and one also reported that earlier menarche is associated with enhanced cognitive functioning.
29 To our knowledge, however, there are no reports on the effects of reproductive events on longitudinal change in cognitive function among postmenopausal women.
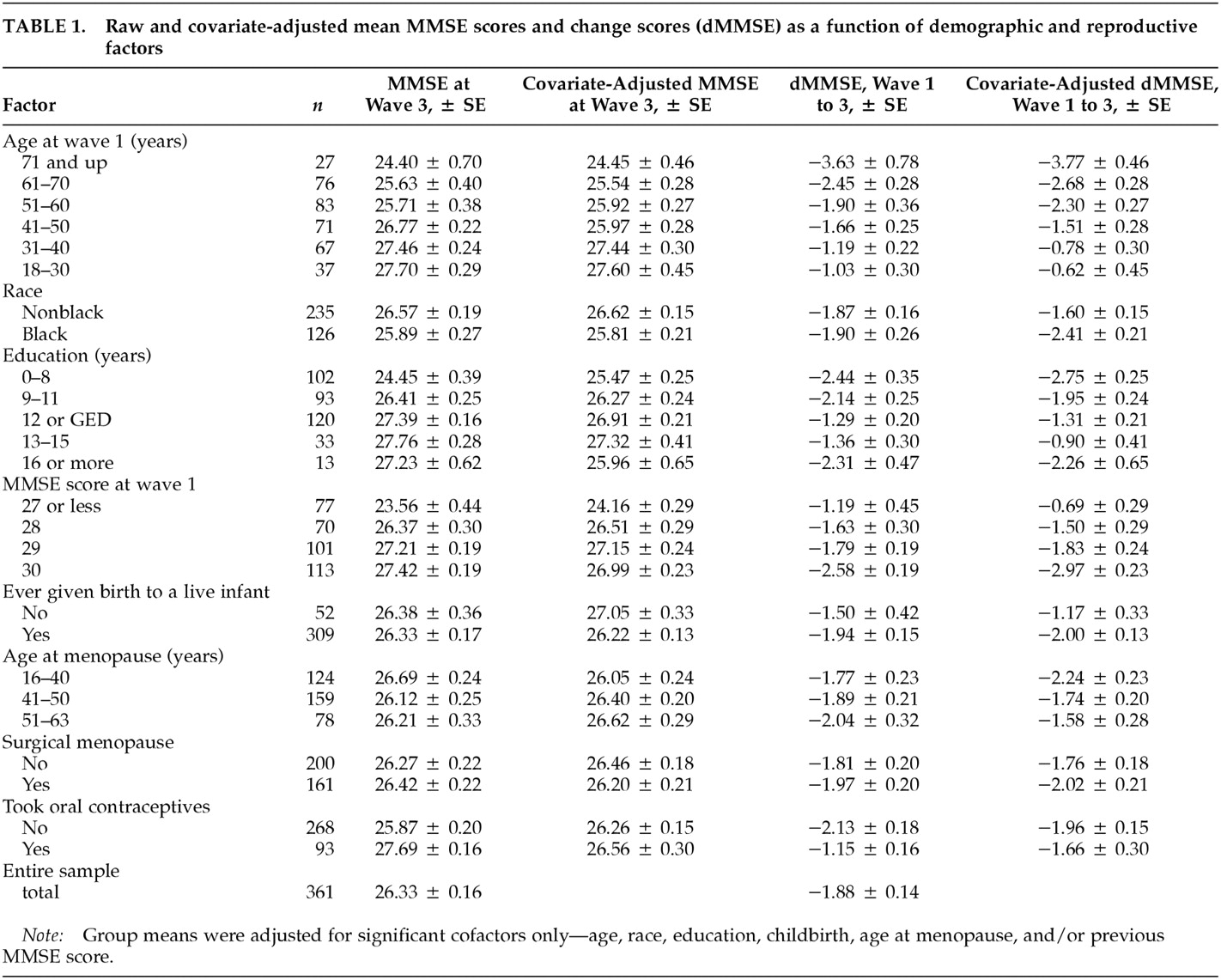
We investigated the hypothesis that reproductive events indicative of increased endogenous estrogen exposure would be associated with less decline in cognitive function. Specifically, we examined the effects of nulliparity, age at menopause, use of oral contraceptive pills, and surgical menopause on change in Mini-Mental State Examination (MMSE) score after a median of 12.8 years in a community-based sample of 361 postmenopausal women. To control for the effects of exogenous estrogens, we included only postmenopausal women who had never received estrogen replacement therapy (ERT). The sample was drawn from a population of adult household residents of East Baltimore surveyed in 1981 and between 1993 and 1996 as part of the Epidemiologic Catchment Area study (ECA). We hypothesized that nulliparity and later age at menopause would be associated with less cognitive decline.
DISCUSSION
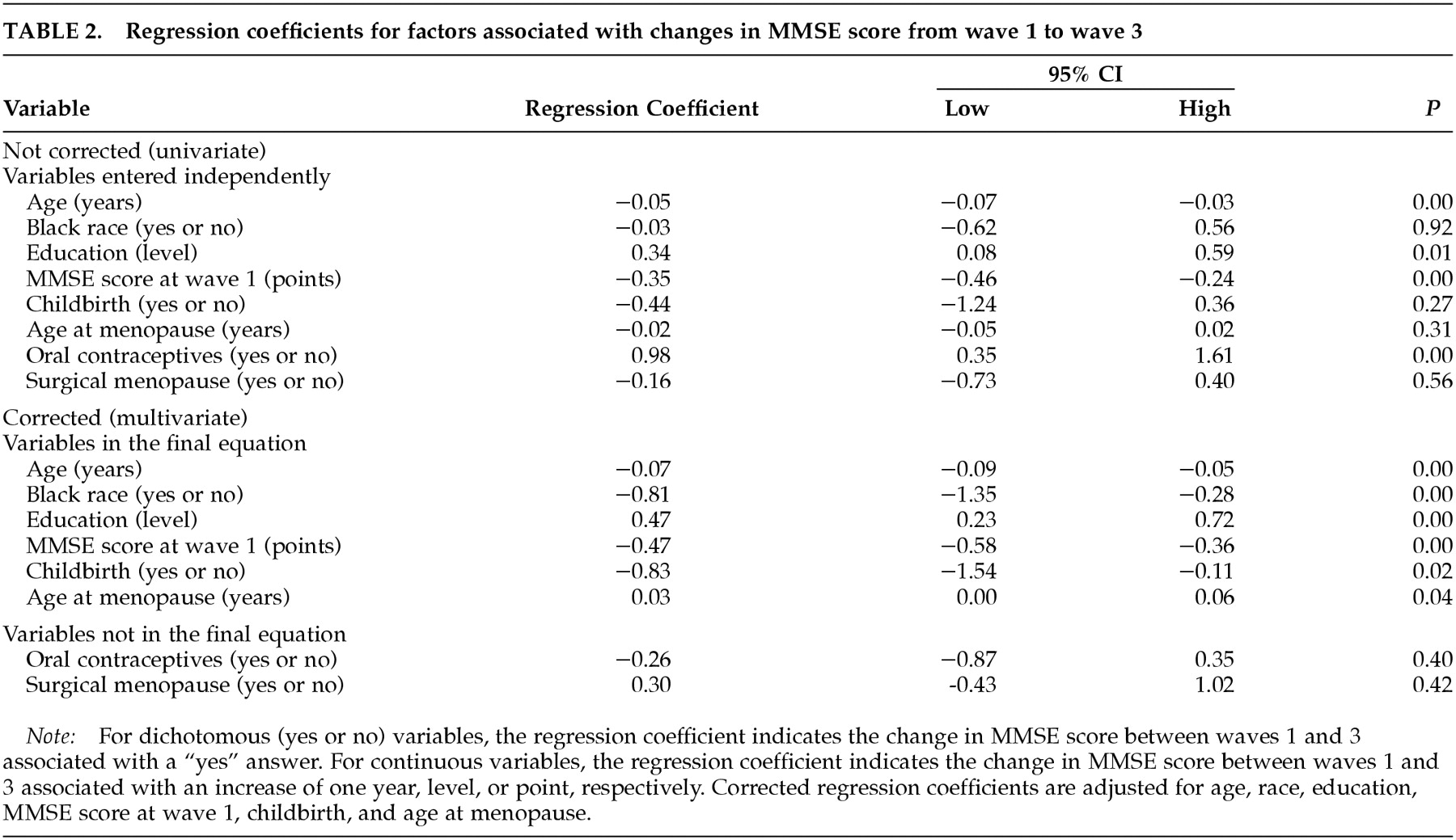
We investigated the effects of reproductive factors indicative of increased lifelong endogenous estrogen exposure—age at menopause, nulliparity, oral contraceptive use, and surgical menopause—on longitudinal change in MMSE scores in a community-based sample of 361 postmenopausal women who had never received ERT. The primary findings of interest were that late menopause and nulliparity were associated with less cognitive decline. These results suggest that in addition to affecting a woman's risk for such age-related diseases as osteoporosis and breast cancer, certain reproductive events may influence age-related changes in the central nervous system.
To our knowledge, these are the first published data demonstrating a relationship between parity and cognitive decline. One previous investigation examined parity and cognition in a sample of 87 nondemented postmenopausal women from an Alzheimer's disease research center, 57% of whom were receiving estrogen replacement.
29 A composite measure of global cognitive functioning was significantly correlated with an index score of endogenous and exogenous estrogen exposure derived from a variety of reproductive factors and medication history.
29 No relationship was observed between parity and MMSE score or any other individual cognitive measure, although the authors noted a negative association between age at menopause and a measure of working memory. Our study had greater statistical power to detect an effect of parity and excluded women receiving ERT, which may modulate any detrimental effects of parity on cognitive function.
A relationship between age at menopause and cognitive function has been observed previously in younger samples of women. A study of 103 patients (mean age, 52 years) who were referred to an endocrine clinic for menopausal complaints found evidence of poorer verbal memory associated with early age at menopause and with surgical menopause.
28 Verbal memory was negatively associated with years since surgery and positively associated with age at surgery. Another study showed that cognitive performances in childhood and at age 43 were positively associated with age at menopause.
34The findings presented here suggest that age at menopause predicts the rate of cognitive decline later in life, after age 60. Although we found no evidence of cognitive decline associated with surgical menopause in our older sample of women, we observed the largest declines in cognitive function among women who were ages 16–40 at menopause, almost all of whom underwent surgical menopause. This suggests that early surgical menopause, at least in the absence of estrogen replacement, may be associated with poorer cognitive outcomes later in life.
In this study, the effect of age at menopause became clear only after adjustment for age, education, oral contraceptive use, and other factors. Without these adjustments, early menopause was more closely, although not significantly, associated with less cognitive decline. However, this appears to be an artifact of our data. On average, the women in our sample who underwent menopause later were older at the time of the follow-up assessment than their counterparts who underwent earlier menopause. (The mean ages for the three groups with early, normal, and late menopause were 55.2 ± 1.4, 66.9 ± 1.0, and 69.3 ± 1.1 years, respectively.) Exposure to estrogen has been shown to increase life expectancy.
35 The effect of age at menopause on life expectancy was not the subject of this study, but the greater average age of the late-menopause group might reflect the benefits of increased estrogen exposure for these women. Other explanations are, of course, also possible.
Both age at menopause and nulliparity were associated with approximately an excess 1-point drop in MMSE performance over an average interval of 12.8 years. A 1-point change is considered small and of negligible clinical importance at the individual level, but at the population level the change reflects a sum of normal and abnormal age-related changes. Detectable changes in MMSE scores at the population level are moderately to highly specific predictors of impairment.
33 In our study, however, only total MMSE scores were examined. Some authors have suggested that in normal individuals, the most common errors in the MMSE occur in recall, calculation, and orientation to time. Impairments in language items may be more useful in distinguishing between normal subjects and those with dementia.
33We were unable to differentiate cognitive decline that was due to normal age-related changes from that due to dementia because formal diagnoses of dementia were not made. The effects of nulliparity and age at menopause on dementia are uncertain. Previous studies of the effects of reproductive factors on risk for Alzheimer's disease have produced mixed results.
10–15 Additional research is needed to clarify whether measurable and statistically significant changes in dMMSE associated with age at menopause and nulliparity signify normal or abnormal age-related changes or a combination of the two.
Data for this study were taken from responses in a study commissioned primarily for the purposes of studying mental health.
30 Thus, the questions asked were often geared toward psychosocial aspects of the participants' lives rather than to record a detailed reproductive history. Many of the hormone-related questions that one would ideally like answered, such as age at menarche, pregnancies that did not result in live births, menstrual cycle history, symptoms at menopause, types and effects of reproductive surgery, abortion, lactation, and other possible confounders that could influence hormonal function, were not part of the interview. Age at menopause was judged by self-reports rather than medical records, and no hormone concentrations were measured. Thus, given the number of potential confounders, no definitive conclusions can drawn about why nulliparity and late menopause were associated with less cognitive decline in this population. However, the findings of this study did fit with predictions based on the effects of endogenous estrogens, and thus it is worth speculating on how endogenous hormones might be involved.
Menopause is associated with drops in levels of estrogens, progestins, and androgens, with simultaneous alterations in receptors and in the ratios of these classes of hormones.
36–38 Although confounding characteristics, such as health factors, of women undergoing surgical menopause at a young age may account for the observed cognitive decline, demonstrations of improved memory among surgical menopausal women after treatment with ERT
24,39 suggest that the relationship could be mediated by estrogen deficiency. Data on ERT in postmenopausal women have been inconsistent, however. If endogenous estrogen were the critical factor underlying the relationship observed in our study between age at menopause and cognitive decline, this might indicate that the lifetime exposure to the hormone, rather than just the postmenopausal replacement, is critical for the neuroprotective effects.
The hormonal changes in pregnancy and childbirth are more complex than those in menopause. A marked increase in estrogen level occurs during pregnancy, followed by a decline after childbirth that lasts about one year; lactation further decreases estrogen levels.
40 High progesterone levels antagonize many estrogenic effects during pregnancy.
41,42 Parity influences estrogen levels later in life.
43–45 During the follicular phases of the menstrual cycle nulliparous women produce higher estrogen levels than parous women.
40,43 The difference in absolute levels of estrogen continues after menopause.
45 Overall, the effect of nulliparity seems to be a higher lifetime exposure to estrogen, which could provide one possible explanation for the effects seen on cognitive decline.
The question of which, if any, hormonal changes underlie the effects we observed will require further study. Some success has been achieved in combining a history of estrogen-altering events into indices
29 and using other physiologic phenomena known to be influenced by estrogen, such as bone mineral density,
28 to predict cognitive ability. Studies that provide both detailed reproductive histories and good longitudinal data on cognitive decline are lacking, however. This study provides an important next step toward the understanding of how reproductive events, hormonal concentrations, and cognitive function are interrelated. Hopefully these findings will spur investigations in which detailed reproductive histories and careful measurements of endogenous hormone levels can be correlated with cognitive changes.
One final note should be made about the possible social implications of these findings. This study reports on an aspect of reproductive function over which women have some degree of control, namely, having children. Although we believe that women should be made aware of all the possible effects their decisions may have, we strongly caution against influencing reproductive choices on the basis of these findings. Our study was limited to postmenopausal community residents who had never received ERT. Study of this population is useful for examining the effects of the natural effects of estrogen; however, it is an unusual population in many respects, and with the increasing popularity of ERT as well as many other sociodemographic changes, this study population is unlikely to accurately represent the future of women currently in their childbearing years. Follow-up studies will be required to confirm the effects of reproductive events on cognitive decline in different populations before definitive conclusions can be drawn.