The relationship between group A β-hemolytic Streptococcus (GABHS) infection and subsequent development of diseases in skin (erythema marginatum, scarlet fever),
1 the heart (rheumatic heart disease),
2 joints (migratory polyarthritis),
3,4 kidney (poststreptococcal glomerulonephritis),
5 and the brain (Sydenham's chorea)
6 has been documented in the medical literature for decades. Streptococcal peptides stimulate specific lymphocytic immune responses,
7 and there is an association of B lymphocytes with a unique surface alloantigen and susceptibility to rheumatic heart disease (RHD).
8 Humoral and/or cellular immune responses, initially mounted against GABHS cross-react to various cardiac tissue-specific epitopes.
9,10 T lymphocytes also infiltrate cardiac tissue.
11,12Published evidence indicates a possible etiological link between GABHS infection and a subset of obsessive-compulsive disorder (OCD),
13–16 Tourette's syndrome (TS),
13,16–19 and autism,
20 and suggests that antibodies that develop against a subgroup of
Streptococcus pyogenes cross-react with human brain tissue in genetically susceptible children. Swedo et al. coined the acronym PANDAS (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections) to represent these disorders. The PANDAS criteria are: 1) presence of OCD or tic disorder, 2) onset between age 3 to puberty, 3) episodic course with dramatic symptom exacerbations, 4) association with GABHS infection, and 5) motoric hyperactivity.
21 The PANDAS concept has recently been reviewed by Murphy et al.
22 and Swedo et al.
23,24 and challenged by others who conclude that currently there is insufficient evidence to establish an association between GABHS and PANDAS.
25–28 These authors argue that there is not clear-cut evidence for concordance of acute rheumatic fever (ARF) and PANDAS, no evidence of an increased incidence of ARF among family members of PANDAS cases, and no clear-cut association of PANDAS with GABHS infections. Further, they argue that antineuronal antibodies have not been found in all TS cases and antineuronal antibodies have been found in a substantial number of control subjects. They also point out that majority of TS cases do not meet PANDAS criteria and doubted if there is room to implicate nongenetic factors as being significant in the pathogenesis of TS.
The mechanism for anti-GABHS antibody cross-reactivity to brain tissue is attributed to molecular mimicry. Susceptibility to instigating an autoimmune reaction in response to an environmental trigger (GABHS or possibly other infectious agents) has been ascribed to a genetic predisposition. Genetic and environmental influences involved in the pathophysiology of rheumatic fever (RF) and PANDAS have not been elucidated, however, recently published papers provide intriguing clues (see below) that suggest that streptococcal superantigens play a crucial role. (The hypothesis that streptococcal superantigens play a critical role in the pathogenesis of OCD and PANDAS was originally advanced by K.A. Williams, co-author of this article, and is currently under analysis.)
The goal of the present paper is to review: 1) microbiologic characteristics of GABHS, 2) immune responses to GABHS as one of the possible etiologies of PANDAS and other neuropsychiatric disorders, and 3) the D8/17 antibody as a potential marker of disease and/or risk for development of PANDAS.
Microbial Characteristics of Group A β-Hemolytic Streptococcus
Group A β-hemolytic streptococcus, or streptococcus pyogenes, is an extracellular, gram-positive bacterium. GABHS is a strictly human pathogen and is responsible for a wider variety of disease than perhaps any other bacterial species.
30 There are over 100 serotypes of GABHS, some of which demonstrate selective tissue tropism; Class I strains are rheumatogenic, and Class II strains are nonrheumatogenic.
31 Recent genome sequencing of a Class I strain of GABHS
32 has determined that this organism has 1,752 predicted protein-encoding genes, including 40 putative virulence-associated genes and microbial molecular mimicry-associated genes.
32An important virulence factor of GABHS, the M protein, protrudes from the surface and interferes with phagocytosis by neutrophils.
33 The M proteins of rheumatogenic strains include epitopes that cross-react with cardiac and other host tissues (trophomyosin).
34,35 Class I strains have M proteins with a C-repeat domain region lacking in M proteins of nonrheumatogenic serotypes.
36 Rheumatogenic GABHS strains are primarily tropic for the throat and cannot produce lipoproteinase, which is characteristic of skin strains.
36 M types of GABHS suspected to cause PANDAS, if different from the ones that cause RF, are not known. Sequence analysis and protein prediction of GABHS virulence factors may provide evidence in support of a neural tissue-specific autoimmune phenomenon in the development of PANDAS.
Immune Responses to GABHS in the Etiology of PANDAS
Poststreptococcal diseases occur after a latent period of 1–4 weeks following a GABHS infection.
30 Established diseases that follow poststreptococcal infections include acute glomerulonephritis and RF with possible complications of RHD and Sydenham's chorea (SC). The latent period before disease implies that a hypersensitivity response to GABHS infection may be responsible for causing disease, rather than the direct effect of disseminated bacteria. Thus, an acquired autoimmune response to pharyngitis caused by a rheumatogenic strain of GABHS may result from bouts of infection by additional rheumatogenic strains that contain the same cross-reactive epitopes.
36 Nephritis is more common after a skin infection, while rheumatic fever is more commonly preceded by infection of the upper respiratory tract. The majority of acute pharyngitis cases are of viral etiology; only 5%–30% of cases are infected with GABHS.
37–39The association of preceding streptococcal infection with development of PANDAS has implicated GABHS as a candidate for initiating a parallel neurospecific autoimmune phenomenon. The relationship between GABHS and PANDAS, however, remains unclear. Certain M protein epitopes have been shown to cross-react with heart tissues, synovium, and brain.
3 Evidence of an interaction between M protein-induced antineuronal antibodies and cognate brain tissue (
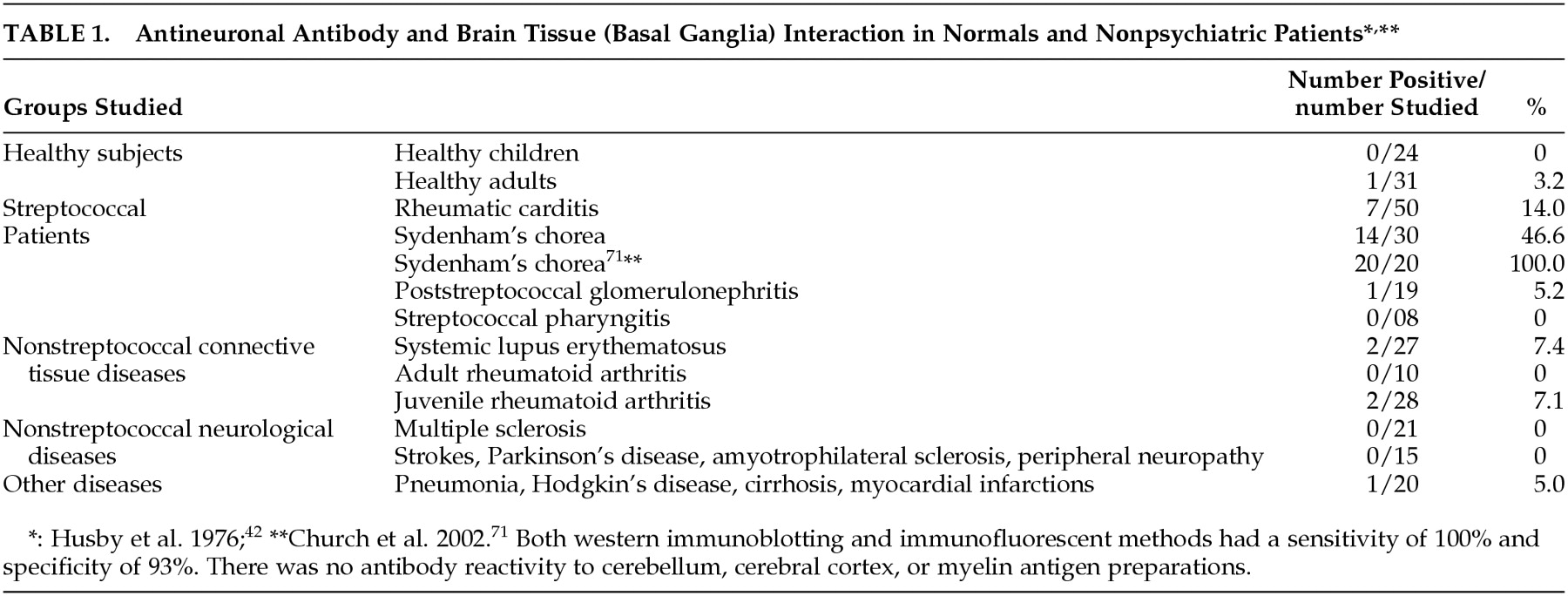
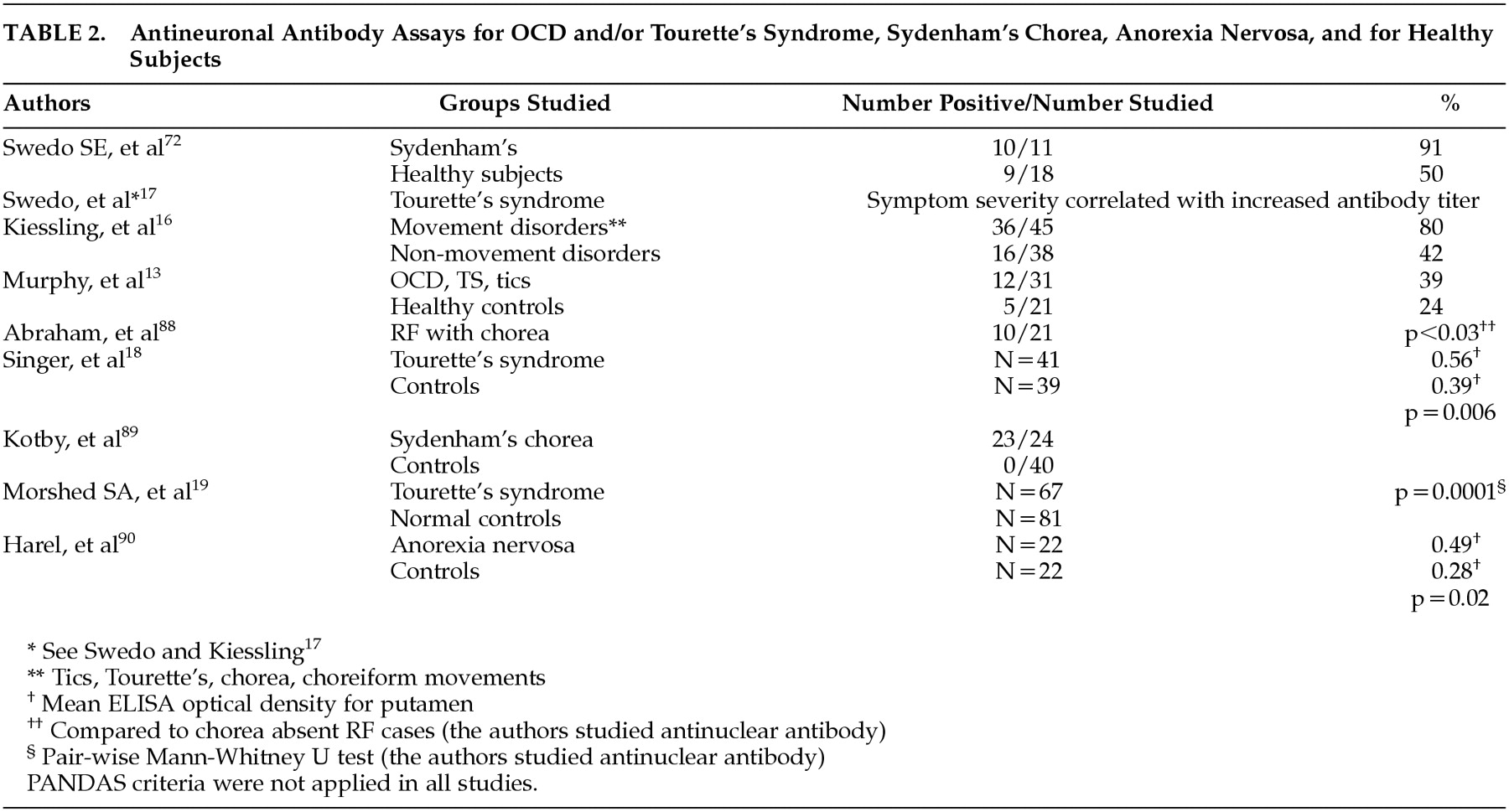
Table 1 and
Table 2) have led investigators to link certain neurological and psychiatric symptoms to a possible autoimmune reaction within the brain. Preliminary evidence also suggests that heterologous M antigens trigger different antineuronal antibodies, each with a unique binding pattern in the brain. The role of B lymphocytes and streptococcal M protein-induced autoantibodies in PANDAS and SC has been examined recently.
16,34,35,40–43Most recently, a prospective study provided convincing evidence establishing a temporal relationship between GABHS infections and PANDAS.
44 In this study, authors proposed the role of GABHS-associated toxins in the development of pathophysiology of PANDAS instead of the role of traditional antibodies. GABHS-associated toxins act as superantigens within the host. By binding both the Class II major histocompatibility complex molecules on antigen presenting cells (HLA Class II) and specific Variable-β regions on the T cell receptor, superantigens can undermine immune function.
45 Allelic changes within the HLA Class II can lead to proliferation of specific T cell clones at a far higher orders of magnitude than what would be expected in the absence of superantigens.
46,47 These expanded T cells interact not only with the M protein epitope but also cross-react with the epitope within the host tissues.
48,49Successful plasma exchange therapy in childhood OCD cases provides supporting evidence of an antibody-mediated etiology of disease.
50,51 Conversely, no response to plasmapheresis was reported in a limited number of children with OCD of a non-PANDAS origin.
52As mentioned previously, both the B cell-mediated mechanisms and T cell infiltration in heart and inflammatory cytokines in the CNS have been noted in RHD and OCD patients.
11,53–57 A recent study showed increased cell-mediated response in CSF of early-onset OCD patients but not in patients with schizophrenia.
58 The cytokines involved in a cell-mediated immune response include IL-2, IFN, and TNF, which are responsible for activating T cells and macrophages.
59 These results suggest that cell-mediated immune mechanisms participate in the pathogenesis of early onset of OCD in addition to the humoral (cross-reactive autoantibodies) mechanisms mentioned previously. Autoantibodies present in PANDAS patients may be the consequence of, rather than the cause of, PANDAS. It is possible that destruction of cells exposes previously masked epitopes that lead to the development of autoantibodies.
D8/17 Monoclonal Antibody as a Potential Marker for PANDAS
D8/17 is a monoclonal antibody developed against a B lymphocyte surface antigen by repeatedly injecting B cells from confirmed RHD patients into mice.
8 Preincubation of the D8/17 antibody with the B cell line originally isolated from an RHD patient markedly diminished binding of D8/17 antibody to cardiac muscle. In Western blot analysis D8/17 antibodies did bind to recombinant type M6 protein, vimentin, and myosin.
60 These findings indicate that this B cell alloantigen may be related to contractile proteins present in heart, skeletal, and smooth muscle and may also share epitopes with some components of GABHS. The D8/17 is not in the subset of autoantibodies potentially endogenous to these patients, nor is it a natural antibody present in these patients; rather, it was generated in mice as a monoclonal antibody. Therefore, since the D8/17 antibody is not produced by patients, it is not involved in the establishment of immune memory.
Subsequent studies have investigated expression levels of this B cell surface alloantigen on lymphocytes from RHD and PANDAS patients by using flow cytometry or indirect immunofluorescence. (Flow cytometry is a method for quantitating components or structural features of cells primarily by optical means. In this case, it can detect specific proteins on the surface of cells.) In RHD patients, the number of B lymphocytes with this unique surface alloantigen is increased regardless of the status of the disease, suggesting that this alloantigen may potentially serve as a trait marker.
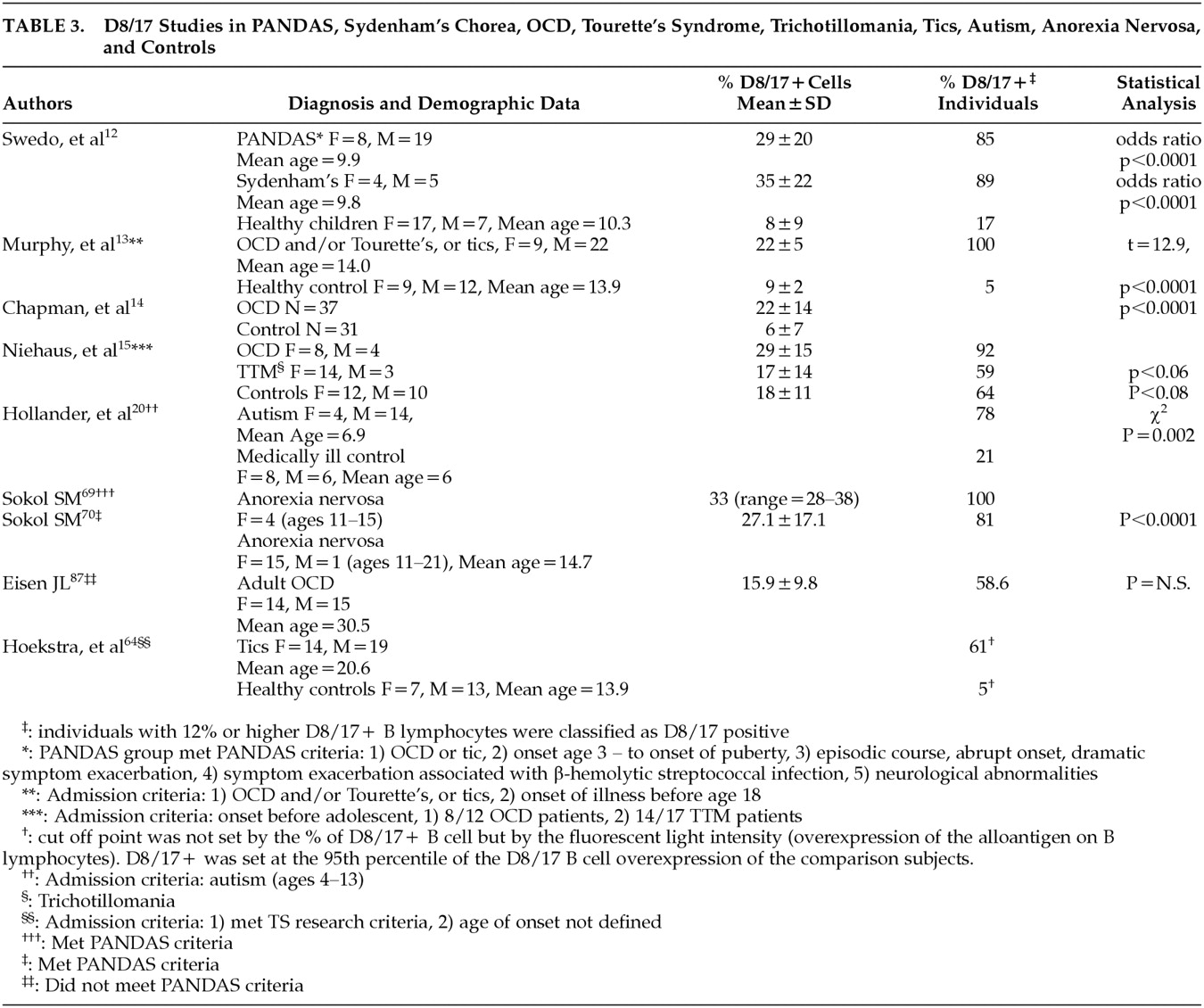
60 Studies demonstrate that the percentage of B lymphocyte populations that express this marker is increased in PANDAS patients (
Table 3).
The control groups within the studies showed consistently lower rates of test positive individuals. One recent study that used flow cytometric analysis showed a sensitivity of 77% and specificity of 87% for correlation of D8/17 staining with OCD and TS.
14 Earlier studies in RF cases showed that D8/17 binding is both highly sensitive (false negative in 0%–10% in RF) and specific (false positive in 5%–15% in healthy volunteers).
60,61 In two studies, D8/17 binding was positive in over 60% of RHD cases and in less than 15% of the control groups.
62,63In these studies, a D8/17 positive B cell count that reached 12% of total B lymphocytes or higher was considered positive. An issue that has been raised concerns the use of isotype control to account for nonspecific antibody binding. Hoekstra et al.
64 used an irrelevant IgM monoclonal antibody (MOC 32) as a negative control. In this study, D8/17 positivity was ascertained by mean fluorescence intensity. A shift in mean fluorescence intensity in test patients was reported, while no significant elevation in the percentage of B cells staining positive for the unique D8/17 responsive antigen was detected. This study concluded that B cells as an aggregate population did not segregate into D8/17-positive and D8/17-negative groups; rather, each B cell showed increased expression of the alloantigen.
64 As stated above, Murphy et al.
65 confirmed this finding in their recent report.
In the Swedo et al. study,
12 all subjects met PANDAS criteria, while in other studies
13,20 prepubertal onset with defined diagnoses (OCD, TS, autism) were sufficient to meet the study inclusion criteria. The rates of positive D8/17 test results, however, were not significantly different in these studies. Since it is estimated that about 10% of OCD is presumed to meet PANDAS criteria
66 it may be that the latter two investigations enrolled study subjects who failed to meet PANDAS criteria. Incidentally, in the study by Hollander et al.,
18 the increased repetitive behaviors among autistic children were associated with greater D8/17 expression. These early findings suggest that the PANDAS criteria may undergo modifications depending on the outcomes of the future studies. Presently, there are no published data indicating that OCD or TS patients who fail to meet PANDAS criteria are D8/17 negative. Although limited in scope, D8/17 has also been examined in anorexia nervosa
67–70 and trichotillomania.
15The discrepancies found among published studies cited above may be due, in part, to the differences in the methods used in these studies. For example, Murphy et al.
65 stressed the importance of using a specific dilution of D8/17 (1:1000) to detect its specific epitope.
Until the molecular characteristics of this B cell alloantigen or its monoclonal antibody (D8/17) are known, the conflicting findings reported above, and the difficulties found working with this IgM monoclonal antibody, may remain elusive.
Discussion
Although the significance of D8/17 testing for OCD is in question, the relationship between recurrent streptococcal infections and development of sudden onset of OCD in a subset appears firmly established.(44) Streptococcal antibodies from SC subjects (and by inference OCD) that bind to basal ganglia but not to the rest of the brain tissue
71 support above findings. Currently, superantigens from a specific streptococcal strain or strains are believed to play a crucial role in the expansion of specific clones of T cells that target a specific epitope on the M protein as well as host tissue (molecular mimicry)
48,49 (see also Immune Responses to GABHS in the Etiology of PANDAS section).
Regarding the D7/18 monoclonal antibody, eight recent studies investigating D8/17 positivity on the surface of B cells demonstrated elevated D8/17-positive cases in a subset of neuropsychiatric disorders that includes PANDAS, SC, OCD, TS, trichotillomania, tics, autism, and anorexia nervosa (
Table 3). In these studies, investigators did not use uniform standardized laboratory procedure, thus the reliability of these study results is in question. For example, recently Hoekstra et al. stressed the importance of using an isotype control to account for nonspecific antibody binding
64 and Murphy et al. recommended a specific dilution of D8/17 (1:1000) to detect its specific epitope.
65The observation that obsessive compulsive (OC) symptoms from SC are indistinguishable from OC symptoms found in chronic childhood OCD cases
72,73 has led Swedo et al. to hypothesize that SC and OCD share the same or similar pathophysiology or etiology.
73 Sydenham's chorea (SC) has known pathologic findings (that date back nearly a century) in the corpus striatum,
74 thus the etiology of OCD was presumed to follow corresponding anatomical and/or pathophysiological changes. Swedo et al. have subsequently conducted a series of studies that have redefined a subset of OCD, with an increased rate of OCD in first-degree relatives of PANDAS cases than those reported in the general population,
75 which are distinct clinical characteristics
21 and laboratory findings. Specifically, OCD children were found to have an expanded subset of B lymphocytes staining positive for the D8/17-specific antigen (
Table 3), increased antineuronal antibody titers (
Table 2), and enlarged corpus striatum.
76 The increased basal ganglia size found in the study was similar to that found previously for subjects with SC compared with normal subjects.
77Regarding the relationship between PANDAS and GABHS infection, a recent study reported that an association between antistreptococcal antibodies and OCD/tics might have been confounded by the presence of attention deficit hyperactivity disorder (ADHD).
27 The authors enrolled subjects ages 7 to 55 regardless of the status of GABHS infection. In this study, elevated antistreptolysin-O (ASO) and anti-DNase B titers were associated with ADHD but not with tics or OCD. Increased ASO and anti-DNase B titers, however, are nonspecific measurements of recent and repeated streptococcal infections, do not indicate immunity, and are not pathognomonic of complicated streptococcal infection. In the case of RF, when ASO and anti-DNase B levels are increased, the diagnosis of rheumatic fever has always been considered only presumptive.
36 It is true that subjects in the above study were younger than the subjects in some OCD/tics studies, and thus may have affected the study outcomes.
Although OC symptoms from OCD and SC are similar, pathogenic strains responsible for SC may be different from serotypes associated with PANDAS. The PANDAS concept was introduced, in part, to allow for the characterization of particular strains of GABHS that are prone to the induction of PANDAS in the susceptible host.
21 A relevant publication demonstrated two different immunofluorescence staining patterns in human brain by two separate streptococcal strains, one with M5 antigens and the other with M6 antigens. M5 staining was confined to the caudate region (SC) but M6 staining was more diffuse.
42 These antibodies detected a number of brain antigens, but prior absorption by M5 or M6 antigens abolished the reactions.
43 The findings suggest that heterologous M proteins trigger different antibrain antibodies that engender different symptoms or clinical courses.
A recent study has reported that serum antibodies from OCD patients bind to human brain tissue.
78 In this study, antibody binding was most pronounced in the basal ganglia although binding in other regions was also detected.
78 Postmortem studies have demonstrated evidence of vascular inflammation and perivascular infiltration,
79 as well as neuronal damage within and outside of the basal ganglia tissue in children with SC.
74,79,80 Vascular inflammation within the CNS was also reported in living SC patients.
81,82 The neuronal damage is postulated to be associated with brain tissue antigens interacting with streptococcal antineuronal antibodies.
41,71 Absorption with GABHS membranes abolished the immunofluorescent staining pattern in neuronal cells, indicating that the antineuronal antibodies were induced by GABHS.
41 Although patients with systemic lupus erythematosus (SLE) also have elevated titers of antinuclear antibodies that bind to caudate cells, the antinuclear antibodies found in SLE patients are not absorbed by streptococcal antigens.
41 The tissue-specific staining pattern was evidenced by the fact that only isolated neurons abolished the immunofluorescent-staining pattern.
41The association of SC as a constituent of RF with RHD sequela is also well established.
83 In RHD, GABHS M protein induced antibodies interact with cardiac antigens.
10,34,35 In SC, the GABHS-induced antibodies appear to interact with brain tissue antigens (
Table 1 &
Table 2). Antineuronal antibody binding to brain tissue, projected from the time of original GABHS infection, and development of SC are temporally mismatched. Whereas antigen-antibody binding occurs within minutes, development of SC often takes several months after GABHS infection.
84Additionally, if antineuronal antibody binds to basal ganglia tissue the manner through which it crosses the blood-brain barrier is not clear. Does an inflammatory process allow antineuronal antibodies to cross the blood-brain barrier? Swedo and Kiessling advanced several plausible possibilities that may allow such antibodies to cross the blood brain barrier.
85In SC, OCD, or TS, antineuronal antibody titers have been shown to correlate with symptom severity.
16,17,73,85,86 Whether putative antineuronal antibody binding to brain tissue is associated with changes in underlying pathophysiology is unclear. Published reports also suggest that OC symptoms precede chorea and that OC symptoms resolve before SC symptoms.
73 The temporal sequence of OC and SC symptom emergence and resolution as well as the ability of an underlying immune mechanism to effectuate clinical manifestation remains elusive.
In summary, emerging evidence strongly suggests an involvement of streptococcal superantigens in the pathogenesis of RF
48 and Kawasaki disease.
49 Changes in HLA class II alleles appear to affect superantigen functions, dramatically leading to the proliferation of specific T cell clones,
46,47 which appear to target cardiac and vascular tissues.
48,49 The pressing need at this time is to examine the role of superantigens from M18 strain (known to cause RF) in SC and PANDAS. We speculate that subsets of other psychiatric disorders such as anorexia nervosa, body dysmorphic disorder, and ADHD may also share pathophysiology similar to that of RF. Findings from these studies, if affirmative, would have a profound impact for the practicing physicians in psychiatry and pediatrics.