Both selective serotonin reuptake inhibitors (SSRIs) and amesergide (AMSG; LY237733) appear to produce an antidepressant effect through their action upon the serotonin (5-HT) system. They differ, however, in that AMSG is a reuptake inhibitor as well as a highly selective antagonist at the 5-HT receptor. AMSG shows affinity at 5-HT
2 receptors similar to that seen for other potent 5-HT
2 receptor antagonists such as ketanserin, ritanserin, and setoperone. In contrast to these agents, however, AMSG has low to negligible effects at α-, β-, dopamine, histamine, γ-aminobutyric acid, benzodiazepine, and muscarinic receptors.
1The literature reports contradictory findings for depression and treatment associated cerebral blood flow (CBF) changes. Increases in dorsal frontal and dorsal anterior cingulate hypoperfusion and hypometabolism with antidepressant therapy have been reported in a number of studies.
2–4 In contrast, decreases in the ventral anterior cingulate blood flow were found in response to desipramine,
5 electroshock therapy,
6 and fluoxetine.
7 The specific effects of the treatment with 5-HT
2 receptor antagonists on CBF in major depressive disorder (MDD) have not yet been reported.
Some of the discrepancies among previous studies may be related to clinical considerations, including diagnostic criteria, inclusion of both bipolar and unipolar subjects, demographic characteristics, illness severity, and medication status. In a number of studies, one difficulty is the alignment of scans from one subject to the next in the absence of anatomical data, which increases the possibility of obscuring significant findings or finding artifacts due to misalignment.
We used [Tc99m]hexamethylpropyleneamineoxime (HMPAO) single photon emission computed tomography (SPECT) images of CBF co-registered with anatomical magnetic resonance (MRI) imaging to compare the changes in cerebral perfusion in response to treatment with SSRIs or AMSG.
METHOD
Nineteen subjects meeting DSM-IV criteria for MDD were recruited from referrals from other psychiatrists and also from the community by advertisement. Inclusion criteria were a current episode meeting criteria for MDD, right-handedness, and no other medical illness potentially affecting the brain. A psychiatrist experienced in the use of the Diagnostic Interviews for Genetic Studies, a structured interview with high reliability,
8 assessed all subjects clinically. Exclusion criteria comprised a current or past neurological disorder, head trauma, uncontrolled hypertension, myocardial infarction or ischemia, diabetes, Cushing's disease, steroid use, drug/alcohol abuse, and use of any psychotropic medication within 3 weeks prior to inclusion in the study. Depression severity was rated using the Hamilton Depression Rating Scale
9 (HAMD) on the day of the SPECT study. All patients were then randomized to treatment with AMSG 15–30 mg daily (Eli Lilly, Inc.) (
n=10) or SSRI 20 mg daily (
n=9) (fluoxetine
n=6 or paroxetine
n=3). In the case of AMSG this was part of a double-blinded, placebo-controlled study. In the case of SSRI it was an open-label study. After 12 weeks, depressed subjects were scanned again during their final week of antidepressant treatment. Clinical response was defined as a posttreatment HAMD score <12 or a 50% decrease compared to the initial HAMD score.
Written informed consent was obtained from all subjects after the procedures had been fully explained. The Human Studies Committee and Radioactive Drug Research Committee of Washington University School of Medicine approved the study.
SPECT
All SPECT CBF scans were performed on a Prism 3000 triple-headed scanner fitted with a high-resolution low-energy collimator (Picker International, Cleveland) after the injection of 16 mCi of HMPAO. The imaging protocol acquired 120 brain images parallel to the orbitomeatal line in 40 steps with 360° rotation of the camera. Reconstruction of SPECT images used a ramp filter to yield transverse slices with a matrix of 128×128×128 pixels and voxel size=2.8×2.8×2.8 mm.
MRI
MRI scans were performed on a Magnetom SP-4000 1.5-T imaging system (Siemens, Iselin, N.J.). A magnetization prepared rapid gradient echo (MPRAGE) acquisition was used to acquire anatomic images, which consisted of 128 contiguous 1.25 mm thick sagittal slices. Scanning parameters were TR=10 msec, TE=4 msec, inversion time=300 msec, flip angle=8°, matrix= 256×256 pixels, voxel size=1×1×1.25 mm.
SPECT-MRI Co-Registration
The MRI images were manually segmented using ANALYZE (Mayo Clinic, USA) to remove the scalp, skull, and meninges, then resized to isotropic voxels. The segmented magnetic resonance (MR) brain images and SPECT images were co-registered using Automated Image Alignment (AIR) software.
10 The MR images were transformed to a reference MRI in Talairach atlas space.
11 SPECT scans were then rotated, translated, warped and resliced according to the transformation matrix generated from combining the two types of co-registration.
Statistical Parametric Mapping (SPM) Analysis
Statistical parametric mapping 96 (Wellcome Department of Cognitive Neurology, University College, London) was used to detect significant (P<0.001) regional changes in CBF between the baseline and treatment scans for both AMSG and SSRI groups combined and in contrast to one another. Correction of global differences and detection of voxel-by-voxel changes were performed using an analysis of covariance. Single photon emission computed tomography data was filtered with a 12 mm full width three-dimensional Gaussian filter at half maximum prior to processing.
A chi-square statistic was calculated to determine whether the gender distribution across the two groups was significantly different, and a Student's unpaired t test was used to determine differences in age and baseline HAMD score.
RESULTS
Subjects in SSRI and AMSG groups were similar in gender (male/female ratio was 4/5 and 4/6, respectively; χ
2=0.038,
P=0.84), age (41.7±11.0 years, and 45.6±13.4 years, respectively) (
t=0.7, df=17,
P=0.49) and baseline HAMD scores (22.8±6.3 and 21.3±4.4, respectively) (
t=0.6, df=17,
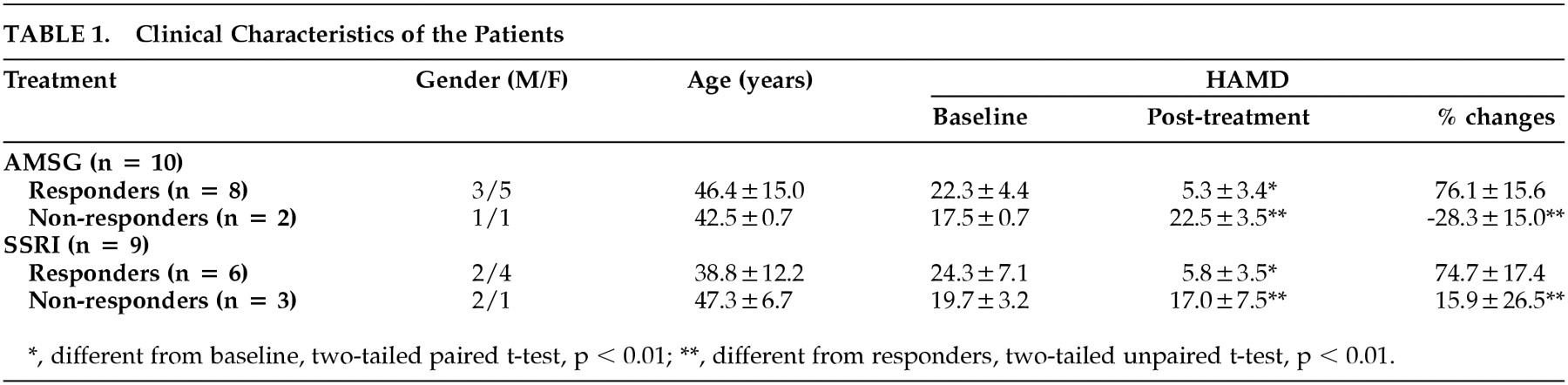
P=0.55). In AMSG group, 80% of subjects improved clinically after the treatment (posttreatment HAMD was 5.3±3.4, a decrease from initial value was 76%±16%) as compared with 67% in the SSRI group (posttreatment HAMD score and decrease from initial value were 5.8±3.5 and 75%±16%, respectively.) (Shown in
Table 1.)
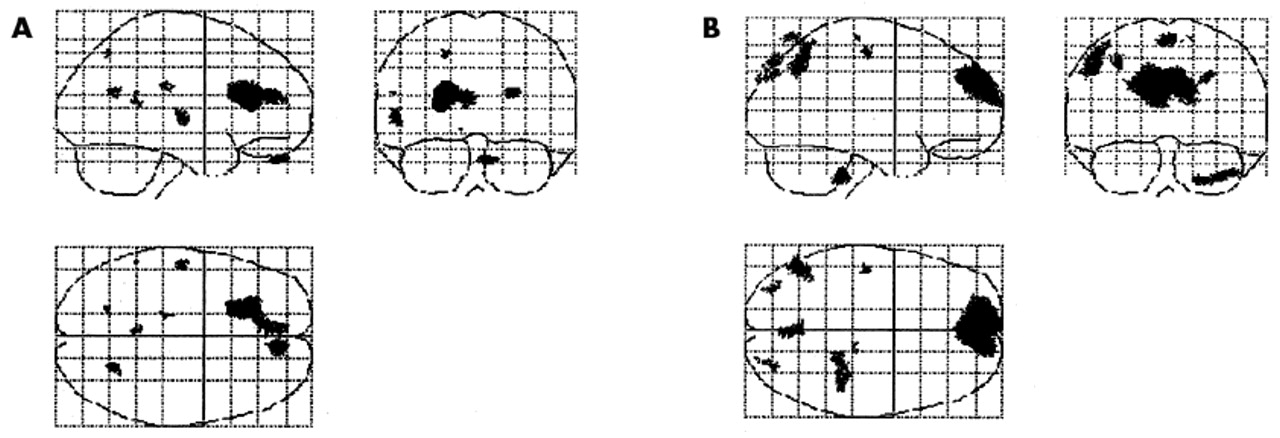
Statistical parametric mapping was used to detect CBF changes between baseline and posttreatment scans in the SSRI and AMSG responders. Regions that increased significantly following treatment with AMSG or SSRI included the left anterior cingulate gyrus (coordinates: −21, 22, 20), which extended towards the midline (covering the middle anterior cingulated gyrus [coordinates:−5, 45, 20]), the left superior temporal gyrus (coordinates:−57, −17, 6) and the orbital prefrontal cortex (coordinates: 11, 51, −29) (
Figure 1). Regions that decreased significantly following treatment with AMSG or SSRI included the left inferior frontal gyrus (coordinates: 65, 17, 22) and left medial temporal gyrus (coordinates: 70, 0, −19). Regions in which AMSG differed from SSRI with a significantly higher increase than SSRI in blood flow following treatment included the medial prefrontal cortex (coordinates: −10, 65, 26), precuneus (coordinates: 0, −77, 65) and the right inferior parietal cortex (coordinates: −46, −60, 50) (
Figure 1). There were no regions in which SSRIs increased blood flow significantly more than AMSG.
DISCUSSION
The primary effect we found in this study was that treatment response to both types of serotoninergic antidepressants is associated with increased CBF in the left and mid anterior cingulate, left superior temporal gyrus, and orbital prefrontal cortex. This change in regional neural activity may be due to the change in mood state or to a common serotonergic effect. Anterior cingulate regions participate in autonomic, affective, and motivational behaviors (rostral and ventral regions), pain perception, attention to action and response selection (dorsal regions), and they have unique reciprocal connections not only between their rostral and dorsal parts, but also with selective dorsal neocortical and ventral paralimbic areas.
12 Anterior cingulate regions and their projection sites are the areas where blood flow and metabolic changes were seen in previous studies
2,12,13 and these changes were improved in MDD patients who responded to antidepressant treatment
3,12 or electroconvulsive therapy.
14We did not analyze the nonresponders group separately due to few numbers of patients. However, Mayberg et al.
12 demonstrated that the metabolic activity in anterior cingulate region discriminated eventual responders from nonresponders and suggested that this area is necessary for the normal integrative processing of mood, motor, autonomic and cognitive behaviors, all of which are disrupted in depression.
We found response to AMSG treatment to be associated with increased CBF in the medial prefrontal cortex, which was not seen in the SSRI treated patients. Decrease in medial prefrontal CBF associated with the cognitive impairment of depression or so-called depressive pseudodementia was demonstrated by Bench et al.,
13 and clinical recovery from depression resulted in CBF increase in these regions.
15 Numerous reciprocal connections between anterior cingulate, dorsolateral prefrontal, and medial prefrontal areas were demonstrated in primate studies,
16 and prefrontal areas are considered to be the sites of convergence for limbic inputs and to serve the function of integration of thought and emotion.
17To our knowledge this study is the first report of medial prefrontal blood flow changes in MDD patients successfully treated with a 5-HT2 receptor antagonist. Studies of other similar drugs (e.g., nefazodone) would be informative in determining whether this effect extends to other 5-HT2 antagonists.
ACKNOWLEDGMENTS
This study was supported in part by a grant from Eli Lilly, Inc., and NIMH grants MH-01370 and MH-58444 to Dr. Sheline, NIMH grant MH-54731 to Dr. Mintun, and grant RR-00036 from the NIH Division of Research Resources to the General Clinical Research Center at Washington University School of Medicine.