Core to current neuropsychiatry is the concept that parallel circuits originating in multiple areas of the cerebral cortex underlie much of human behavior. Emotion, memory, and behavior are widely studied in relation to circuits that begin and end in either the frontal lobes or the limbic system.
2 The cerebellum was not traditionally incorporated into these circuits. As case reports and series emerge from the literature noting nonmotor symptoms in patients with isolated cerebellar lesions, the cerebellar role in cognition, mood, and behavior is becoming more visible.
In the past, the anatomy of brain circuits was inferred by combining case reports, imaging data, autopsy results, and many experimental animal studies in which anterograde and retrograde tracing techniques were used to label neurons and their projections. Recently exciting new virus-based tracers have been introduced that are able to cross the synapse and thus allow specific labeling of neurons in sequence for the first time. With these new methods it is now possible to trace neuronal connections across several synapses, allowing mapping of parallel circuits in unprecedented detail. These studies provide strong evidence for the segregated nature of parallel frontosubcortical circuits and support the addition of the cerebellum to these pathways. Very importantly, they are beginning to open a window to the functional topography of the cerebellar cortex and the deep cerebellar nuclei.
Corticocerebellar Circuits and Virus Based Tracers
Most studies delineating neuronal connections within the brain are done in animals. The rapid progress made in the past few decades in identifying these connections has been primarily based on the use of anterograde and retrograde tracers such as horseradish peroxidase and the dextran amines.
3 These substances have made it possible to probe the fine structure of the brain, but are limited to labeling of single neurons. Circuits are inferred by combining the results of multiple experiments. With the recent development of tract-tracing methodology based upon the use of neurotropic viruses it is now possible to directly visualize neurons that are linked together in a circuit. These viruses are taken up into neurons where they replicate and pass from neuron to neuron at synaptic connections in a time-dependent manner. The apparent rate of transport is to some extent dependent on the rate of viral replication and the time required for
trans-synaptic passage of the virus. Neurons infected with virus are identified using conventional immunohistochemical labeling with antibodies to the virus. Evidence to date supports the view that virus transport is not restricted to particular types of synapses, but rather labels both excitatory and inhibitory connections.
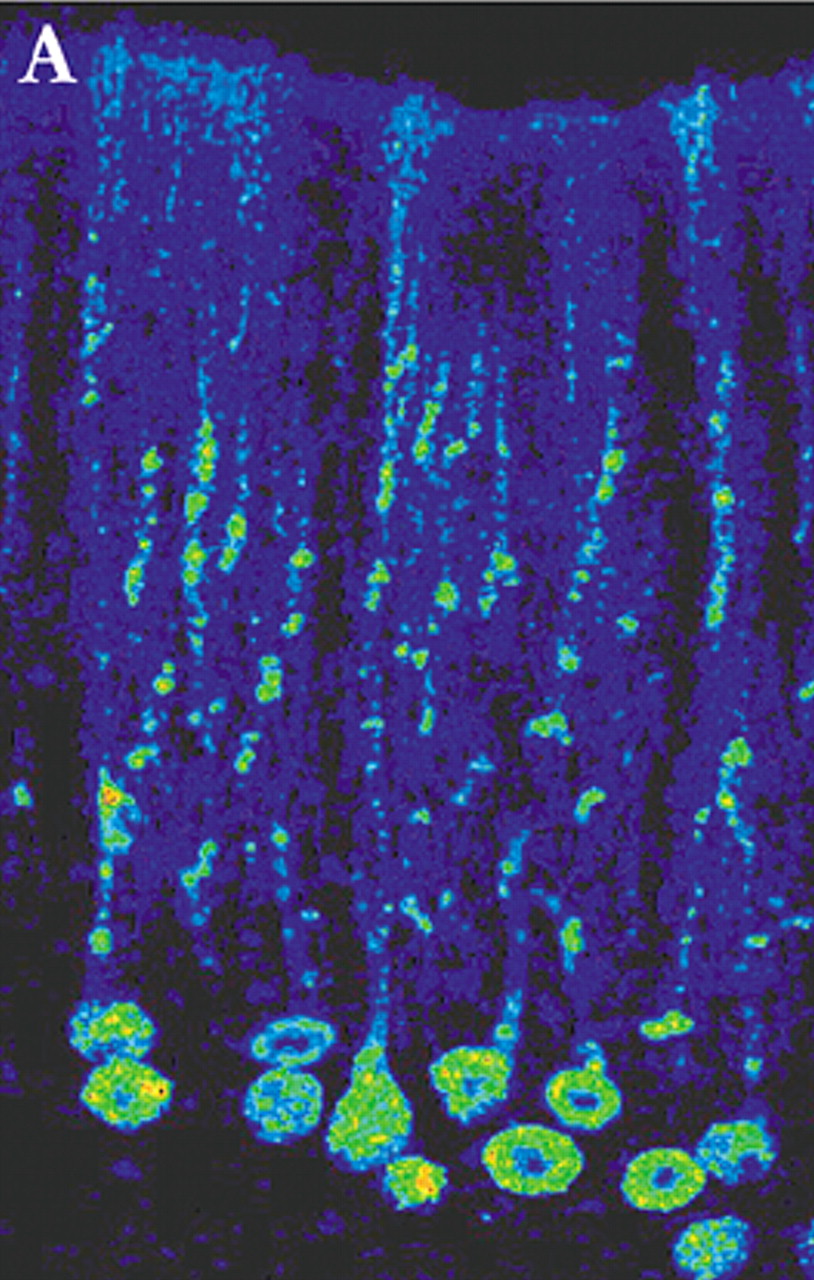
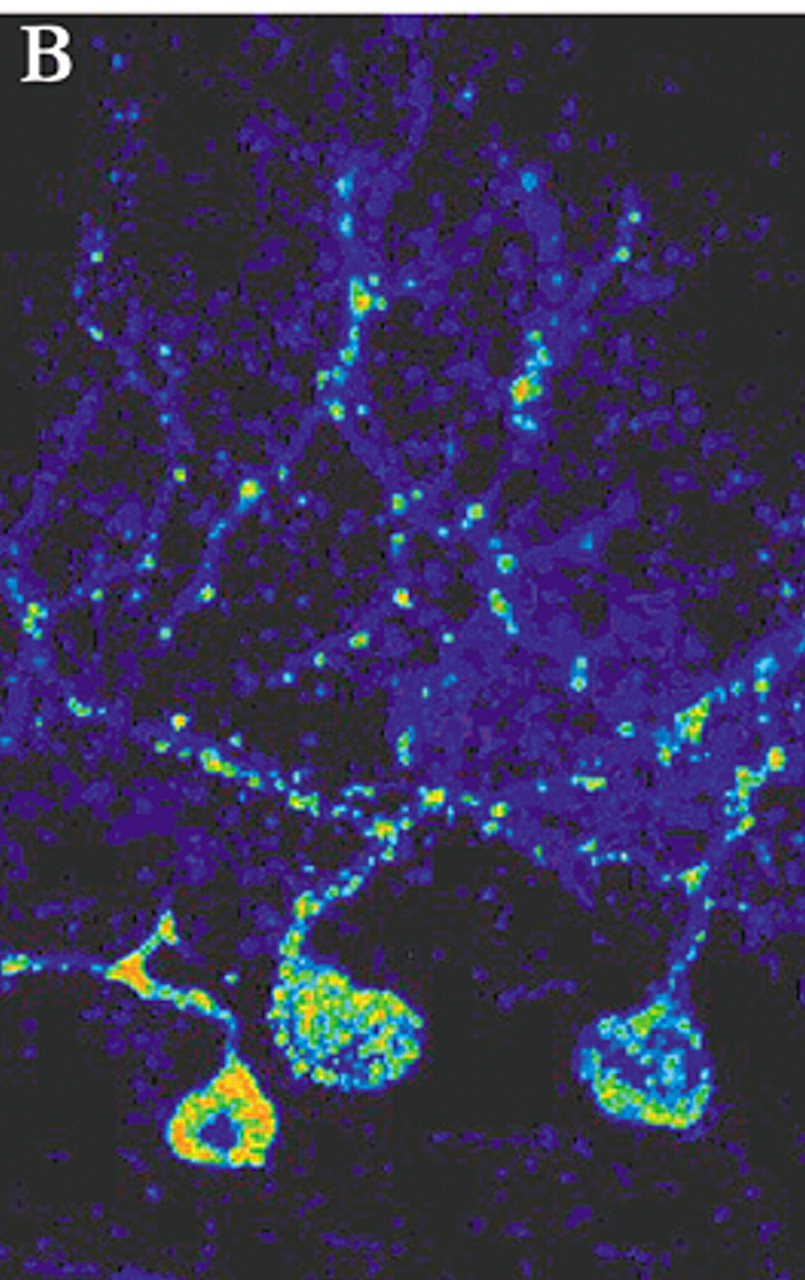
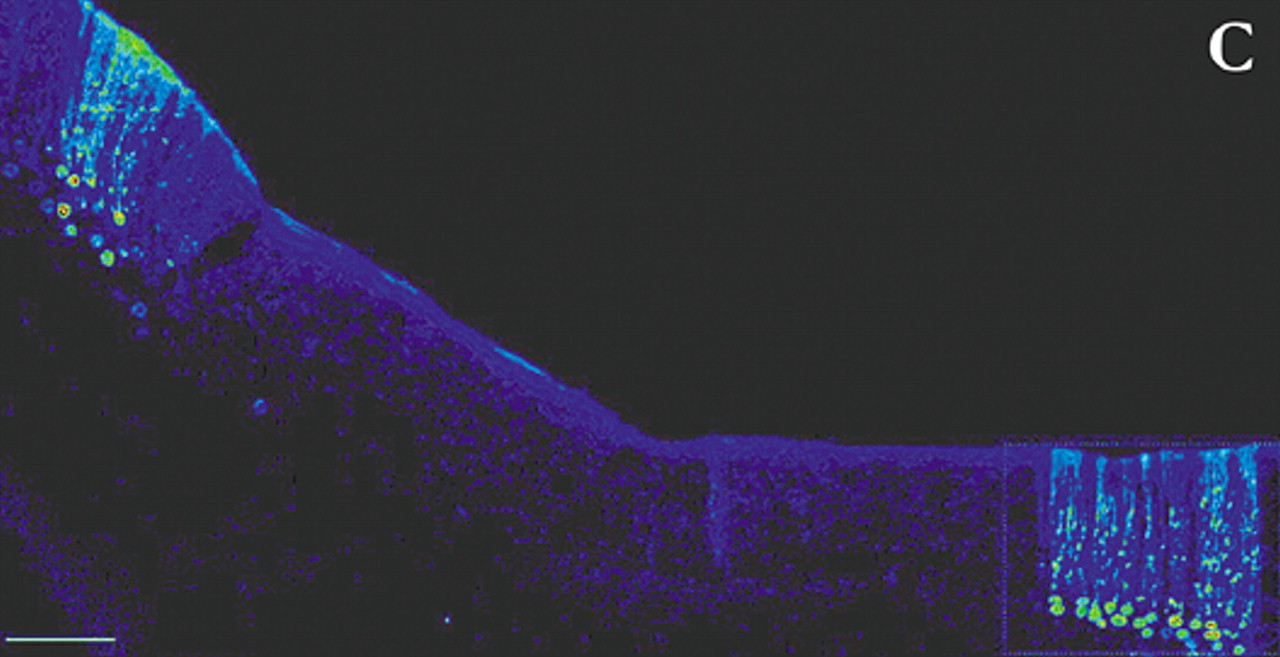
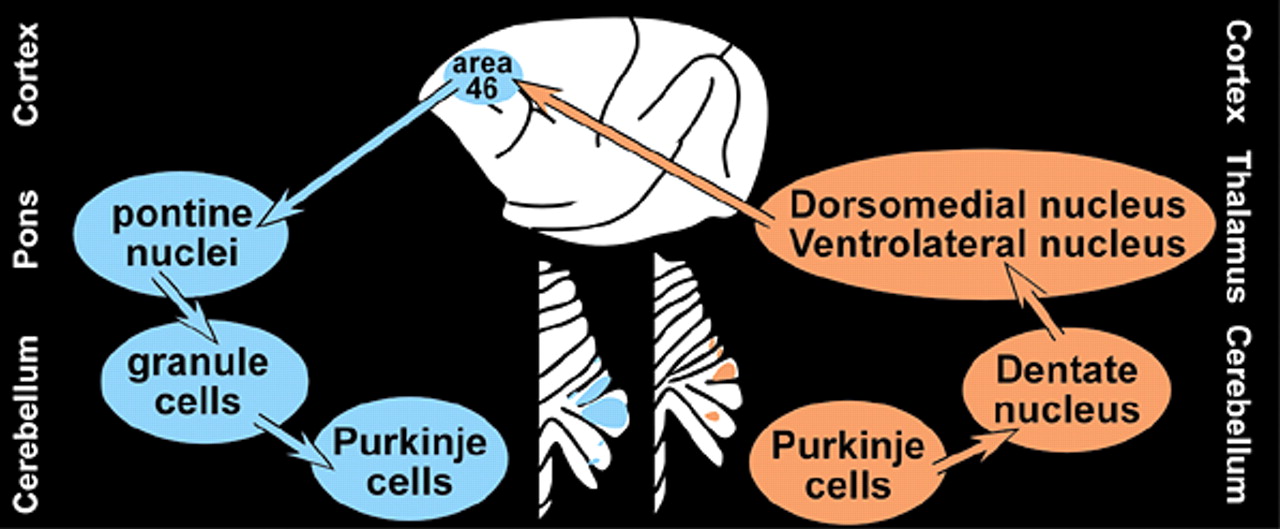
4 By appropriate selection of survival time after injection, multisynaptic pathways can be labeled with great precision. For example, by 2 days after injection of rabies (retrograde tracer) into primary motor cortex (M1), neurons in the ventrolateral nucleus of the thalamus were labeled. By 3 days, label was also found in the dentate nucleus of the cerebellum. At 4 days, it was present in cerebellar Purkinje cells. By 5 days, cerebellar granule cells were labeled (
Figure 1a,
Figure 1b, and
Figure 1c ).
5In primates, commonly used viruses are herpes simplex virus type 1 (HSV1) and rabies. Herpes simplex virus type 1 is transported in either the anterograde (H129 strain) or retrograde (McIntyre-B strain) direction, depending upon the strain used.
6 At longer survival times HSV1 causes neuronal lysis.
4 There is the potential that release of the virus into the extracellular space could complicate interpretation of the labeling pattern. Glial proliferation is also seen, as well as occasional glial labeling, although these do not seem to present major problems.
4 Rabies virus is particularly useful because it specifically infects neurons (glial infection is quite rare) and it does not cause cellular lysis.
5 Several strains of rabies have been evaluated. Although the rates of transport varied, all were transported only in the retrograde direction. Theoretically, both viruses could be combined in a single experiment in order to define an entire circuit. Unfortunately, the presence of HSV1 inhibits uptake and transport of the rabies virus, so they cannot be used together.
1 Coadministration of other tracers, such as wheat germ agglutinin (WGA), may also interfere with uptake of the rabies virus.
It has been known for many years that the cerebellum receives input (via the corticopontocerebellar pathways) from multiple cortical regions including motor, premotor, posterior parietal, cingulate and prefrontal cortices.
7 Now it has been demonstrated that the cerebellum projects (via the dentatothalamocortical pathways) back to these same areas. A series of studies in nonhuman primates utilizing neurotropic viruses (rabies and HSV1) to demonstrate the parallel segregated nature of these reciprocal connections was recently reviewed.
1 In brief, rabies virus (CVS-11 strain, retrograde tracer) or HSV1 (H129 strain, anterograde tracer) was injected into either M1 or the dorsolateral area (46) of prefrontal cortex (PFC). Labeled cells were confined to specific areas of the cerebellum. M1 injections labeled clusters of neurons in lobules IV-VIII, while area 46 injections labeled clusters of neurons in Crus I and Crus II (
Figure 2). Comparison of results with the 2 tracers (anterograde and retrograde) indicated that extremely similar areas within the cerebellum were labeled. Thus it is likely, although not proven, that the circuits are truly reciprocal.
The overall structure of the corticocerebellar pathways is a three-neuron chain in each direction, beginning/ending at the pyramidal cells in the cortex and Purkinje cells in the cerebellum (
Figure 2). Older studies in nonhuman primates using standard anterograde and retrograde tracers indicate that the corticopontine projections from the PFC arise primarily from the dorsolateral and dorsomedial regions, with little or no contribution from ventromedial, ventrolateral or orbital areas.
7,8 Recently, it has been demonstrated that the cerebellum projects back to PFC via the thalamus.
9,10 Neurons within the cerebellar dentate nucleus were labeled following injections of HSV1 (McIntyre-B strain, retrograde tracer) into dorsolateral and dorsomedial PFC (areas 46 and 9). No label was found in the dentate following injections into ventrolateral or orbital PFC (ventral area 46 and lateral area 12). Different regions within both the dentate nucleus and the thalamus were labeled following injections into each of these areas, confirming the segregated nature of these circuits.
9–11 Thus, the functional topography of the cerebellum—how the cerebral cortex maps onto the cerebellum—is starting to be defined.
Cognitive and Behavioral Symptomology
As individuals and groups of patients with similar diagnoses are studied, the clinical evidence for the cerebellar influence on cognition, emotion, and to a lesser extent personality and behavior is beginning to emerge. Several recent reviews have summarized the literature regarding this evidence.
12–14 Rapoport et al. reminds the reader that more than 50% of all the brain’s neurons are located in the cerebellum, yet, it contains only 10% of the total brain weight.
12 This group proposes that the cerebellum has a “balancing, integrating, and stabilizing” effect on nonmotor functions including affect, mood, and cognition. Schmahmann reviews the history of the study of the cerebellum as well as continuing to develop evidence for the “cerebellar cognitive affective syndrome” (CCAS).
13 He describes CCAS as the psychopathology evident after a cerebellar lesion, most commonly after a posterior lobe lesion. He proposes the cerebellar influence on cognition and behavior to be an “oscillation dampener maintaining function automatically around a homeostatic baseline.”
A common method utilized in matching neuroanatomy to function is to study patients with new psychopathology following acquisition of a lesion or after a new neurological condition is diagnosed (
Figure 3). Schmahmann and Sherman used in-depth neurological and neuropsychological testing to evaluate a series of 20 patients with cerebellar pathology.
15 All 20 demonstrated some level of cognitive, visuospatial, or psychiatric symptomology. The authors proposed that a common syndrome appears after lesion to the cerebellum (most commonly and intensely after lesions to the posterior cerebellum and vermis). Cerebellar cognitive affective syndrome consists of a quad rad of executive dysfunction, visual-spatial deficits, personality changes, and linguistic abnormalities. The executive dysfunction includes problems with planning and judgment, set-shifting, and working memory. Other deficits include disinhibition, flat affect, visuospatial disorganization, and dysprosodia. The quad rad together creates a general reduction in overall intellectual functioning/cognition. The patient-group was diagnostically hetereogeneous, but with no history of previous psychopathology. The authors excluded patients with clinical or MRI (MRI, T1 or T2 weighted images) evidence of pathology outside of the cerebellum. However, the patients were imaged before more sensitive MRI techniques such as the Fluid Attenuated Inversion Recovery (FLAIR) sequence were commonly used. Additionally, only 3 patients in the study had functional imaging (2 with single-photon emission tomography (SPECT) and 1 with positron emission tomography (PET)). All 3 had abnormal scans with cortical, basal ganglia, or thalamic hypoperfusion. The authors attribute this to diaschisis, a previously observed phenonomon where hypoperfusion may occur distant from the site of injury.
Executive dysfunction has also been confirmed in several recent studies of patients with focal cerebellar lesions (infarct, tumor, hematoma). In one study, 13 of 15 patients with isolated cerebellar infarcts demonstrated abnormal performance in categorical fluency, naming, attention, language, or visuospatial abilities, when matched against comparison subjects.
16 Most (14/15) had superior cerebellar or posterior inferior cerebellar artery infarcts. The one patient with an anterior inferior artery infarct was one of the two with no cognitive deficits on testing. Magnetic resonance imaging was obtained to rule out noncerebellar lesions. The MRI sequences used were not documented. Single photon emission computed tomography was performed in 10 of the 15. Six demonstrated basal ganglia or frontoparietal hypometabolism (the proposed mechanism was diaschisis). Another study compared patients with either isolated brainstem (45 patients) or cerebellar (37 patients) infarcts (identified from a stroke registry).
17 In unblinded testing, both groups had similar levels of frontal/executive dysfunction to patients with cerebral lesions in addition to either brainstem or cerebellar damage. One more study compared patients with either cerebellar (18 patients) or brainstem (4 patients) infarcts to comparison subjects.
18 The patients with cerebellar infarcts, although without symptoms on neurological examination, had more neuropsychiatric symptoms (working memory and cognitive flexibility) than patients with brainstem lesions. Unexpectedly, visuospatial deficits inversely correlated with employment at 12 months. The patients who were unable to successfully return to previous jobs complained of headache, anxiety, memory problems, fatigue, and irritation. The complexity of these jobs was not described, but the authors noted that disability accommodations were attempted prior to the patients’ failing to function in the work environment. Deficits in working memory and specific aspects of attention were also found in another study of patients with focal cerebellar lesions.
19 These studies support a role for cerebellum in modulating cognitive function.
Another group retrospectively reviewed the charts of 133 patients with cerebellar degeneration or spinocerebellar ataxia (SCA).
20 In cases where only cerebellar pathology was found (74 patients), 18% had cognitive impairment and approximately 20% depression. The extent and uniformity of the patient assessments is unclear. This same group prospectively compared 31 patients with degenerative cerebellar (CD) diseases to 21 patients with Huntington’s Chorea and 29 comparison subjects.
21,22 Results paralleled the retrospective study, with 19% of the CD patients diagnosed with cognitive impairment. Other significant data from the study included an overall rate of 77% of the CD patients having psychiatric pathology (57.7% mood disorder and 25.8% personality change). Limitations of the study included incorporation of patients whose pathology extended outside of the cerebellum, no MRI or functional imaging examinations to exclude patients with basal ganglia pathology, and use of the Mini-Mental State Examination (MMSE) as the diagnostic tool for the cognitive impairment. The follow-up study examined the same patients with a more in-depth neuropsychological battery and confirmed the previous results. The patients with CD had significant executive dysfunction, but minimal loss of new learning or memory.
22Cerebellar neurological impairments in children are less defined with fewer studies available. One retrospective chart review of 15 children with cerebellar tumors found 37% to have expressive language deficits; 37% to have visual-spatial deficit; and 33% to have verbal memory deficits coinciding with visual-spatial or language dysfunction.
23 Thirty-two percent had significant affective dysregulation with all of these having extensive lesioning of the vermis. Fifty-six percent of those with severe vermal lesions (five of nine) had the classic postoperative resolving mutism. The authors noted that the younger children in the study tended to perform better on the testing than the older ones and that some children’s clinical presentation was much worse that the scores indicated. Possible explanations for this included the retrospective aspect of the study, testing difficulties in young children, neural plasticity, or developmental stage at time of surgery.
Although controversial, there is evidence for abnormal vermal size in several psychiatric conditions including attention deficit hyperactivity disorder, autism, and schizophrenia. For example, a recent study examined 155 neuroleptic-naive patients with schizophrenia and compared them with 155 matched subjects.
24 One-fifth of the patients had focal cerebellar neurological signs. These were associated with higher rates of cognitive impairment, smaller total cerebellar volume on MRI, more severe negative symptoms, and poorer psychosocial functioning. A recent case report proposes a key role for the cerebellum in development of a variant of schizophrenia.
14In summary, there is growing clinical evidence for the involvement of the cerebellum in neuropsychological function. However, the specific cognitive deficits evident on testing are still quite variable from patient-to-patient. Both cognitive deficits and noncognitive psychopathology have been reported in patients with traumatic brain injury, tumors, strokes, or degenerative atrophies (e.g., SCAs) of the cerebellum. Numerous single-case reports are published, but few studies exist with large numbers of patients. Several factors make it difficult to draw firm conclusions. Often times the patients in the existing reports are diagnostically heterogeneous. Studies have included patients whose pathology extends outside the cerebellum (e.g., pons or basal ganglia). In addition, secondary processes such as acute hydrocephalus are sometimes present that might have caused or influenced the presentation of the neuropsychiatric pathology. Finally, vascular conditions commonly associated with stroke generally have wide-spread effects in the brain. Thus, caution is needed when considering the evidence for cerebellar influence on nonmotor functions.
Functional Imaging
Functional imaging provides another line of evidence suggesting the participation of the cerebellum in cognitive/emotional functioning due to its engagement along with frontal areas during many tasks (e.g., making of moral judgments, processing of negative emotions, learning to solve the Tower of London task or a pegboard puzzle).
25–28 A developmental study of the areas involved in generating voluntary inhibition of responses found that adults, unlike children and adolescents, demonstrated activity in the lateral cerebellar cortex and dentate nuclei.
29 The authors suggest that the cerebellum may be important for the maturation of voluntary response suppression mediated by prefrontal areas. Cerebellar activation was also found in an attention shifting task, which the authors attributed to response reassignment rather than attentional switching.
30 The results of the previous study suggest that an alternative explanation is engagement of the cerebellum in order to suppress the inappropriate response.
Three studies have used neurophysiological measures to assess the influence of the cerebellum on frontal lobe functioning. Two used low-resolution electromagnetic tomography (LORETA) to assess cortical function in patients with atrophy limited to the cerebellar cortex.
31,32 In one, the intracerebral distribution of both spontaneous electrical activity (measured by EEG) and the midlatency auditory evoked potential (MLR) was measured.
31 Patients had decreased activity in the alpha2 band of the EEG over the left inferior frontal gyrus (BA 45) and the P1 component of the MLR was decreased in the superior frontal gyrus (BA 9). In the other, event-related potentials (ERP) were used to measure frontal lobe activation during a continuous performance task (CPT) in which a motor response was either performed (“Go”) or inhibited (“No Go”) depending upon a cue.
32 Performance did not differ between the groups, but patients had significantly less frontal lobe activation during the No Go condition. In the third study, the relative activity of the left and right PFC (as measured by qEEG) were compared prior to and following repetitive transcranial magnetic stimulation (rTMR) of the cerebellum in normal individuals (5 patients).
33 A shift in activity in the fast gamma frequency band occurred such that activity was significantly higher in the right PFC than the left following stimulation of the medial cerebellum, a reversal of the baseline pattern. Intriguingly, subjects gave unsolicited reports of increased alertness and elevated mood following the stimulation. All of these studies suggest that frontal lobe function is influenced by the cerebellum.