The year 2005 marks the 50th anniversary of presentation of the first cerebral blood flow (CBF) image. An exciting new era opened in 1955 with the publication of “The local circulation of the living brain,” an
ex vivo study of the feline brain using a soluble gaseous radioactive tracer, trifluoroiodomethane.
1 This technique was called autoradiography because images were acquired by laying radioactive brain sections directly onto x-ray film.
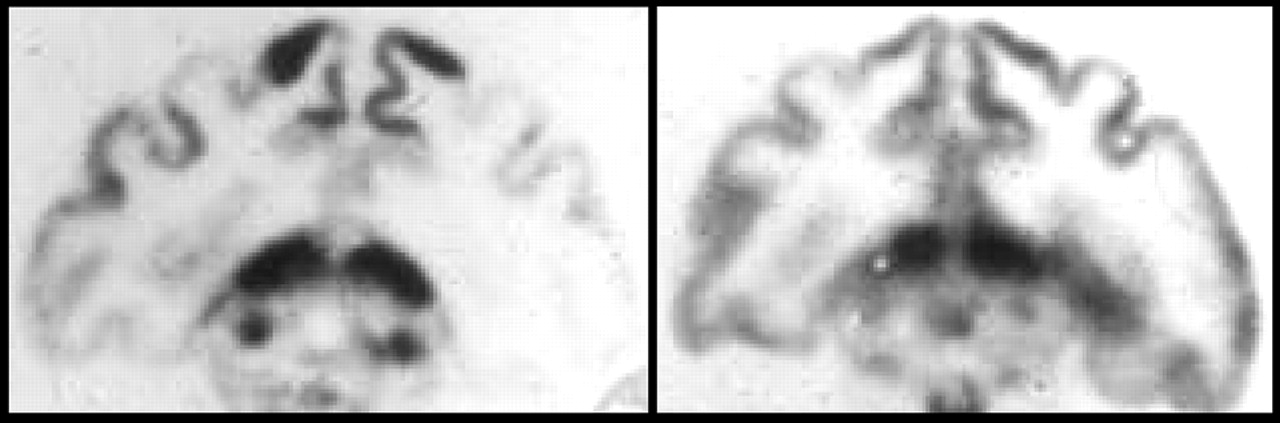
4 The study included CBF images showing clear changes between a baseline (sedated) and stimulated (awake, restrained) state (
Figure 1). This seminal study, which built upon earlier work from the same group demonstrating that CBF is locally regulated, set the stage for the development of the field of functional brain imaging.
5 The volatile nature of the gaseous tracer used initially created challenges.
4 Handling procedures were developed to prevent loss of the gas from tissue. The frozen brain had to be sectioned very rapidly, so sections were necessarily rather thick (∼5 mm). These were stored in liquid nitrogen until placed between layers of x-ray film, surrounded by solid CO
2, and stored in darkness for the 10 hour exposure. Another challenge with this tracer was that its solubility in blood was influenced by both hematocrit and lipid content. The resultant individual differences in blood level of the tracer meant that a calibration curve had to be created for each subject. This group continued to refine the measurement of CBF. They introduced new radiotracers, first
131I-antipyrine, followed by
14C-antipyrine.
6,7 14C-antipyrine had many advantages over previous tracers.
7 It was inert and freely diffusible. With a stable (nonvolatile) tracer it was possible to collect thin (∼20 um) sections and expose the x-ray film at room temperature. In addition, the much longer half-life made it possible to create permanent calibration standards. It also provided much higher resolution images, although exposure times were much longer (∼2–4 weeks).
8 Interestingly, the authors themselves preserved a marked skepticism on the subject during presentation of the initial study. “Of course we recognize that this is a very secondhand way of determining physiological activity; it is rather like trying to measure what a factory does by measuring the intake of water and the output of sewage. This is only a problem of plumbing and only secondary inferences can be made about function. We would not suggest that this is a substitute for electrical recording in terms of easy evaluation of what is going on.”
1 The past half-century of studies have clearly shown that CBF imaging can, in fact, produce valuable information about brain function.
9The critical next step was development of techniques during the early 1970s that allowed imaging of CBF in the living brain, making functional imaging in human subjects possible. This method was initially called positron emission transaxial tomography (PETT), later shortened to positron emission tomography (PET) (because it was possible to reconstruct images in planes other than transaxial).
2 Soon after the invention of PET, the new technique was applied to the measurement of regional CBF using radiolabeled water (
15O-H
2O).
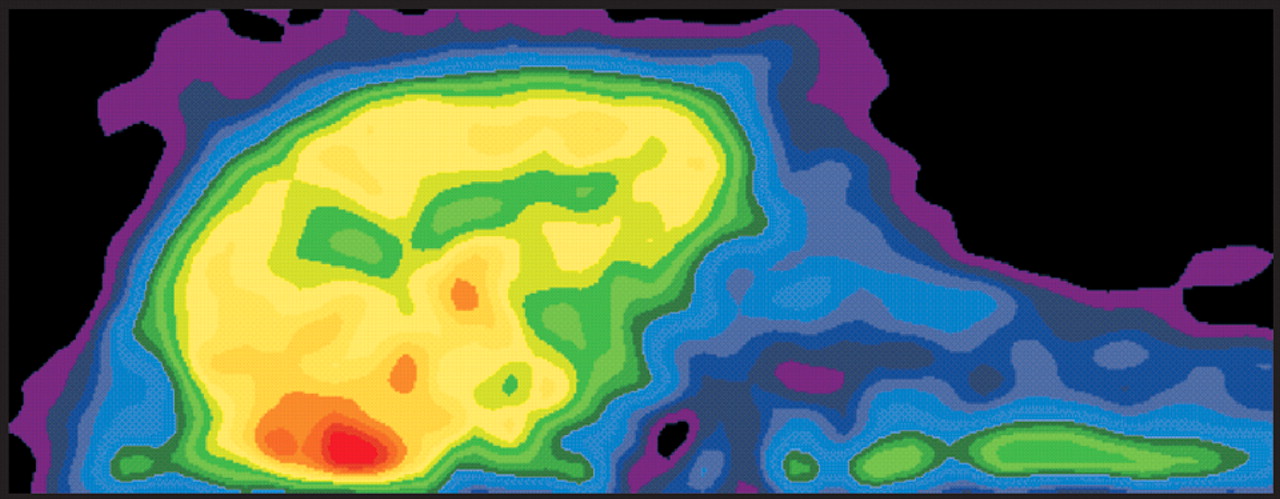
10–12 Advances in physics and engineering have allowed production of PET images of very high resolution (microPET) for animal work (
Figure 2). Animal imaging remains important for both prospective study of experimental models and development of new methods.
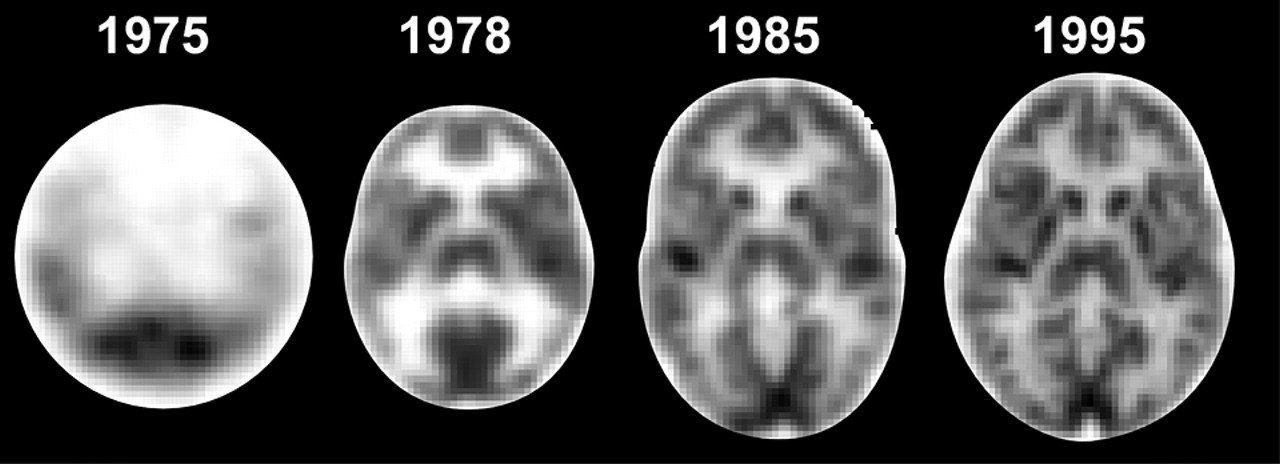
The prototype PET scanner suitable for human imaging was built in 1974, and commercial systems became available in 1978 (
Figure 3).
2 Single photon emission computed tomography (SPECT) was introduced soon after.
13 Both methods were quickly applied to studies of human cognition and of neurological and psychiatric disease, providing insights into processes previously unobtainable.
13–15 It was found, for example, that in normal awake individuals the frontal lobes consistently had the highest CBF and that these regions were responsive to many conditions. In contrast, individuals with schizophrenia often had reduced frontal CBF (hypofrontality) and decreased responsiveness in these regions.
13A major challenge during these early years was development of standardized methods for comparisons within and across individuals and groups. Image resolution was too low for accurate visual identification of anatomical regions. One solution was development of mathematical methods that allowed translation of each individual’s brain scans into the standard stereotactic system used by neurosurgeons.
16 With this conversion in place, it became possible to select regions for analysis based upon the stereotactic atlas of the brain and to identify regions by consultation with the atlas.
16,17 This refinement in technique allowed group studies that had more statistical power, better representation of the population, and examined the whole brain.
18 Imaging of CBF continues to be the major application of SPECT, although receptor studies are becoming more common.
19–21 PET quickly diversified with the development of new radiotracers for a wide variety of measurements including cerebral metabolism and neurotransmitter receptor binding. Now, the majority of PET studies utilize these new tracers.
19–22The most commonly used tracer for PET CBF imaging is radiolabeled water (
15O-H
2O). This tracer has a very short half-life (∼2 minutes), so a bolus injection provides a snapshot of CBF that can be repeated, if required, every 12–15 minutes.
22 This technique has been particularly useful clinically in the study of acute stroke.
23 Decreased perfusion (misery perfusion) is found in areas near the infarct during the first few hours after onset, followed by increases (luxury perfusion) over the first week. PET has been used to monitor the effectiveness of thrombolytic therapy and other interventions. Inhalation of
15O provides several measures, including CBF oxygen consumption, oxygen extraction ratio, and cerebral blood volume (CBV). This is not considered a clinical technique, as it is quite complex.
SPECT regional cerebral blood flow (rCBF) imaging is quite valuable in many neuropsychiatric conditions including dementias, seizures, trauma, movement disorders, anxiety, obsessive-compulsive disorder, and schizophrenia.
24,25 It is widely available as the same nuclear medicine cameras and software that are used for clinical scans of thyroids, prostates, or hearts are also used for brains. Three-dimensional images can be reconstructed as well as the traditional axial, sagittal, and coronal planes of section. The most commonly used SPECT brain blood flow tracer remains
99mTc-hexamethylpropyleneamine oxime (HMPAO) followed by l,l-ethyl cysteinate dimer (ECD). Once injected under quiet dimly lit standard conditions, HMPAO allows for immediate fixation in the brain and scanning up to several hours later.
20 A recent review article provides an excellent in-depth introduction to the science of brain perfusion in SPECT imaging.
26The most widely studied and most common neuropsychiatric use for SPECT imaging is in the differential diagnosis of Alzheimer’s disease (AD) from vascular and other dementing diseases (e.g. Lewy body dementia, fronto-temporal dementia, or Parkinson’s disease).
27,28 A recent metaanalysis found SPECT to have a higher specificity than clinical criteria in discerning AD from other dementias (91% vs. 70%), although clinical criteria have a higher sensitivity.
27 Other applications under extensive study with dementia include the staging of the cognitive decline, mild cognitive impairments in patients likely to develop AD, and new looks at treatment response patterns with the cholinesterase inhibitors.
29–31Localization of seizure foci in epilepsy patients for evaluation of surgical candidacy remains an area of extensive use of brain SPECT. Medication resistant seizures can cause extreme debilitation to patients, and surgical resection after lesion localization can be curative. If the tracer is administered while a seizure is occurring (ictal scan), the ictal focus will have hyperperfusion as compared to the resting or interictal hypoperfusion state.
24,32,33Cerebrovascular conditions such as hypoperfusion, cerebrovascular accidents, transient ischemic attacks, and post-vascular interventions are also imaged with highly successful lesion localization.
24 Other clinical uses for SPECT include identification of areas of abnormal blood flow in vasculitis, multiple sclerosis, and in documentation of poor brain function after trauma.
34–36 Evidence of altered brain function via grossly abnormal blood flow pictures can assist with symptom explanation, diagnostic clarification, and biopsychosocial treatment plan changes in patients with normal structural imaging.
34–36 Investigational uses of SPECT for blood flow in psychiatric diseases include extensive imaging of obsessive-compulsive disorder patients with hyperperfusion patterns and hypoperfusion in schizophrenia, Gilles de la Tourette’s syndrome, and in unipolar depression.
24The PET and SPECT methods for acquiring CBF images provided much of the data that is key to our modern understanding of brain and behavior. These methods continue to be valuable, but clinical applications are limited by their reliance on radioisotope production and administration of ionizing radiation. More recently, several different approaches to imaging CBF have been developed that do not require administration of radiotracers.
Within a decade of the introduction of computed tomography (CT) two methods for evaluating CBF were developed, both based on administration of a contrast agent. The simple addition of a rapid injection of an iodine-containing contrast agent to the CT protocol (first-pass or bolus perfusion CT) allows simultaneous assessment of several parameters, including CBF, CBV, mean transit time (MTT) and blood-brain barrier (BBB) permeability.
37–39 A major advantage of this technique is that it requires no special equipment. The software required to calculate these maps is available from all major CT companies. Its addition to the imaging examination requires only a few extra minutes. First-pass perfusion CT is valuable for examination of acute stroke, providing a rapid method for assessing the extent and severity of ischemia. The combination of CBF, CBV and BBB permeability assessment has also shown potential for grading of cerebral tumors. At present, the major limitation of this technique is coverage. On most CT scanners only a few sections can be obtained. An alternative method of perfusion CT uses a slow administration of contrast agent in order to maintain a steady concentration over a sufficient time to allow imaging of the entire brain. This method, however, provides only CBV measurement.
38The other CT contrast agent that is used to measure CBF is stable xenon gas (Xe).
38,40 When inhaled, this radio-dense, lipid-soluble gas dissolves into the blood and passes into the brain, providing a quantitative measure of CBF. Like perfusion CT, XeCT adds very little time to the exam time. Unlike most methods of imaging CBF, XeCT is truly quantitative and has been found to be accurate even at very low and very high flow rates. An additional advantage is its rapid elimination, which makes repeated scanning under different conditions (e.g., drug challenge) possible. Xe is a narcotic gas, however, and even in the low doses presently used some euphoric or dysphoric side effects are seen. XeCT is valuable in the management of acute stroke, allowing differentiation of the salvageable ischemic penumbra from the core. This is critical information, for if no area within the stroke is still viable, then thrombolytic therapy should not be commenced. Management of severe traumatic brain injury is also enhanced by use of XeCT CBF studies to identify development of conditions (e.g., cerebral swelling) that can lead to secondary brain injury. In February of 2001 the use of xenon as an X-ray contrast agent was temporarily halted by the FDA, pending completion of required studies that are currently under way at many academic medical centers.
Magnetic resonance (MR) imaging also provides two approaches to measuring CBF.
38,39 Like first-pass perfusion CT, one approach uses administration of a contrast agent (this technique is variously called dynamic susceptibility contrast, first-pass or bolus perfusion MR imaging). Unlike XeCT, however, MR contrast agents remain intravascular. In addition, MR contrast agents alter image intensity indirectly by the effect of the contrast agent on surrounding tissues. These differences complicate quantification. A weakness of this technique is that at the present time only approximate measures of CBV, CBF and MTT can be derived. Quantifying the tracer in a given volume of magnetic resonance imaging (MRI) space is not as straightforward as quantification of a radiolabeled tracer with PET. This is an active area of research.
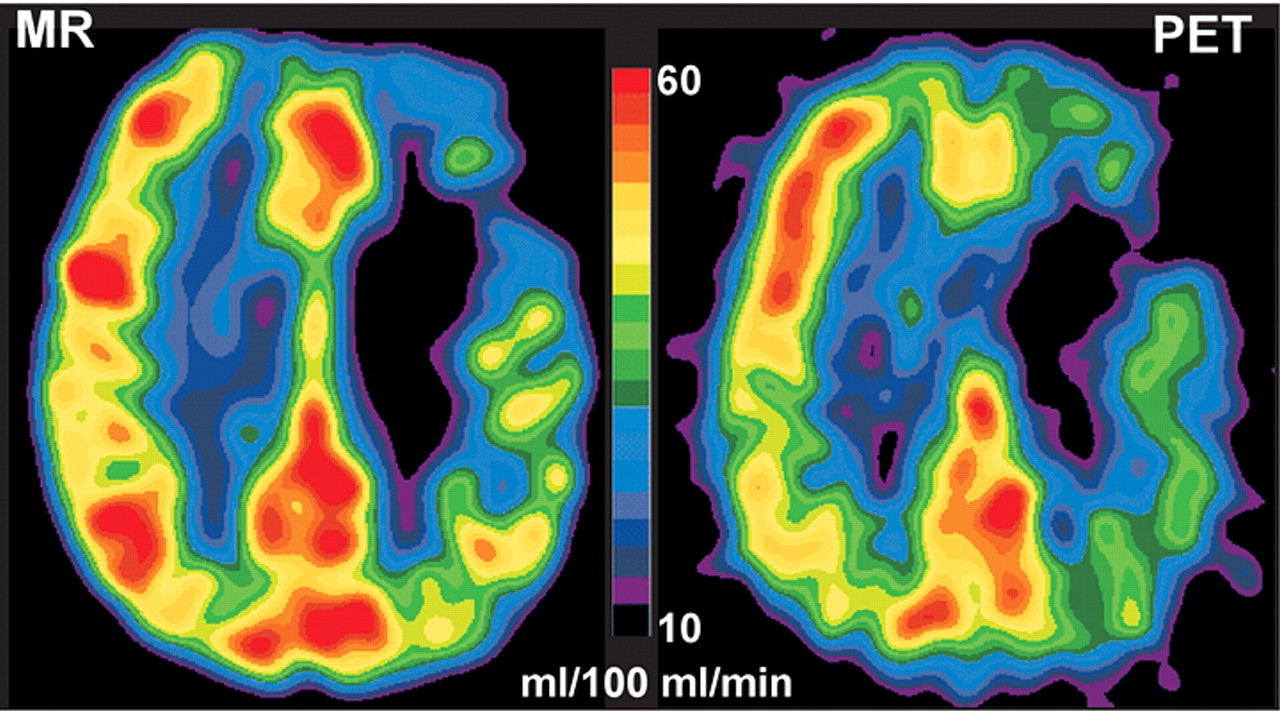
41 Direct comparison studies indicate that there is good qualitative but not quantitative agreement between CBF measurement made with PET and dynamic susceptibility contrast perfusion MR (
Figure 4).
3,42 A major advantage of this technique is the absence of exposure to radiation of any sort. The most common clinical application is evaluation of acute stroke.
38,39 Recent work suggests that it may also be useful in monitoring of multiple sclerosis.
43The other MRI method for imaging CBF does not require administration of a contrast agent; rather water molecules in the carotid arteries are “tagged” by radiofrequency pulses, which change the signal from the water in blood, making the blood itself into a contrast agent.
44 The altered signal is imaged shortly thereafter as the tagged blood flows upward through the brain. This approach is called arterial spin labelling (ASL). Multiple ASL MR imaging methods have been developed. While this way of CBF imaging is still considered an experimental technique, it has great potential for future clinical and research applications. Most importantly, it requires neither exposure to any form of radiation nor administration of a contrast agent, and measures can be repeated as often as required.
The development and application of methods to measure local brain blood flow in living humans revolutionized neuroscience and has captured substantial public interest. Functional imaging has significantly increased our understanding of the emotion and behavior circuits of the brain. As always, caution must be used in interpreting individual data. Many factors can influence functional studies including comorbidities, technical factors, medications, individual patient state, and tracer injection conditions. More extreme care is needed when medicolegal issues arise. One recent review notes the limitations of such imaging in forensic testimony.
45