Catechol-
O-methyltransferase (COMT) is active in the metabolism (breakdown) of dopamine and norepinephrine by catalyzing the methylation of these catecholaminergic neurotransmitters. COMT is likely to have an important role in regulating levels of synaptic dopamine, especially in regions of the brain such as frontal cortex where levels of dopamine transporters are low.
1,2 Genetic factors can influence the amount and activity of different forms of enzymes necessary in the metabolic pathways, as reviewed by Winterer and Goldman.
3 Therefore, genetic factors affecting COMT function are thought to impact catecholaminergic neurotransmitter pathways in this brain region.
3A large body of evidence supports the idea that disruption of the prefrontal cortex impairs cognitive processes, in particular executive functions.
4 Executive functions are those abilities that allow individuals to efficiently and effectively engage in complex goal-directed, planned behaviors, including awareness, initiation, planning, concept formation, organization, goal setting, mental flexibility, goal modification, self-awareness, and self-regulation.
5,6Isolation and structural characterization of the human COMT gene have defined the molecular basis for allelic differences in COMT enzyme activity. COMT Met 158 (Met) is associated with a fourfold decrease in COMT specific activity as compared to COMT Val 158 (Val).
7 As the prefrontal cortex does not express dopamine transporters, another mechanism for synaptic dopamine removal, COMT activity is likely to have the greatest effect on synaptic dopamine levels in this brain region although it is anticipated that altered COMT levels will also affect norepinephrine metabolism in prefrontal cortex. Thus, individuals with the Met allele are expected to have increased levels of endogenous dopamine in prefrontal areas and individuals with the Val allele are expected to have lower levels of endogenous dopamine. Frequencies of the Val and Met alleles are 0.54 and 0.46, respectively, in European Caucasians
7 while the frequency of the Met allele in African and Asian populations is lower than in Caucasians.
8A relationship between COMT Val158Met and executive functioning has been demonstrated in patients with schizophrenia,
9 healthy siblings,
9 and healthy comparison subjects.
9,10 Specifically, Egan et al.
9 found an allele dosage relationship of the Val allele to perseverations on cognitive tasks in schizophrenics, healthy siblings, and healthy comparison subjects. Subjects with the Val/Val genotype were more perseverative on one measure of executive functioning, the Wisconsin Card Sorting Test (WCST), than those with the Met/Met or Val/Met genotype. However, in another small study, genotype effects on WCST perseveration were seen in schizophrenic patients but not comparison subjects.
11 In addition, another recent study by Bilder et al. found a genotype effect in schizophrenic patients for cognitive domains of attention and processing speed, with the Met/Met group performing better.
12 Attention and processing speed are often affected by lesions in frontal systems. Moreover, it is important to note that the cognitive tasks in this study were grouped into four cognitive domains based on prior factor analytic work. Perseveration on the WCST was part of a “general executive and perceptual organization” domain that also included measures of verbal fluency, as well as measures of visual-spatial functioning and visual memory. Examining the raw scores for the WCST demonstrates that the Val/Val genotype group did display the poorest performance, though statistical significance was not reached.
Deficits in executive functioning are often found in patients following traumatic brain injury (TBI).
6 Although TBI often results in injury to the dopamine rich frontal and temporal lobes, the role of COMT polymorphisms in executive functioning has not been studied in this population. We hypothesized that individuals with the Met variant of COMT (less active enzyme leading to increased available dopamine) would perform better on measures of executive functioning than individuals with the Val variant (more active enzyme leading to less available dopamine). To test this hypothesis, results of measures of executive function were compared among the three different genotype groups in the TBI sample: Met/Met, Met/Val, and Val/Val.
RESULTS
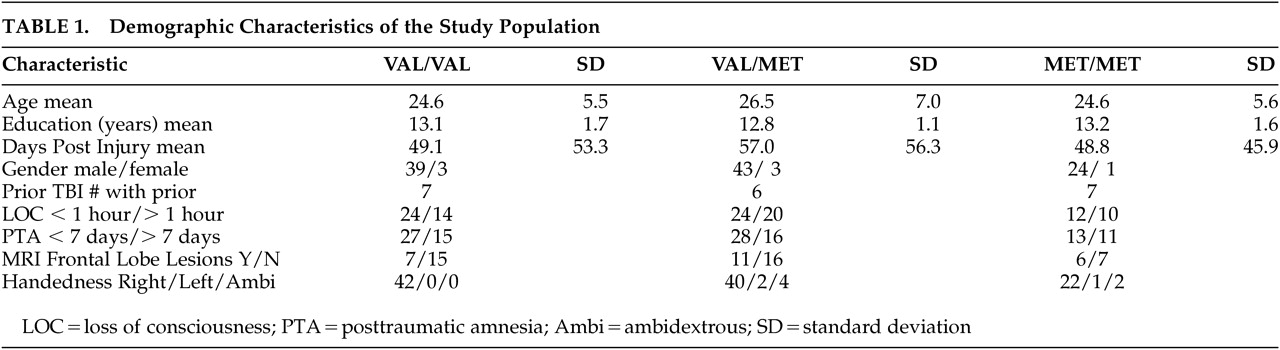
One hundred thirteen individuals were evaluated for the association of the COMT Val158Met polymorphism on aspects of executive functioning following TBI. Characteristics of the study population are presented in
Table 1, where it can be seen that this was a relatively young, predominantly male sample. Severity of injury was dichotomized based upon duration of loss of consciousness (LOC) (≤1 hour or >1 hour) and length of posttraumatic amnesia (PTA) (≤7 days or >7 days). While not reaching statistical significance, mean scores for the tests of executive function were in the predicted direction with the more severe injury group demonstrating poorer performance than the less severe injury group. MRI frontal lesion data available on 62 of the 113 participants did not show any differences between genotype groups. Of the total sample, 42 were homozygous Val/Val, 25 were homozygous Met/Met, and 46 individuals were heterozygous Val/Met. The total Val/Met allele frequencies were 0.58/0.42.
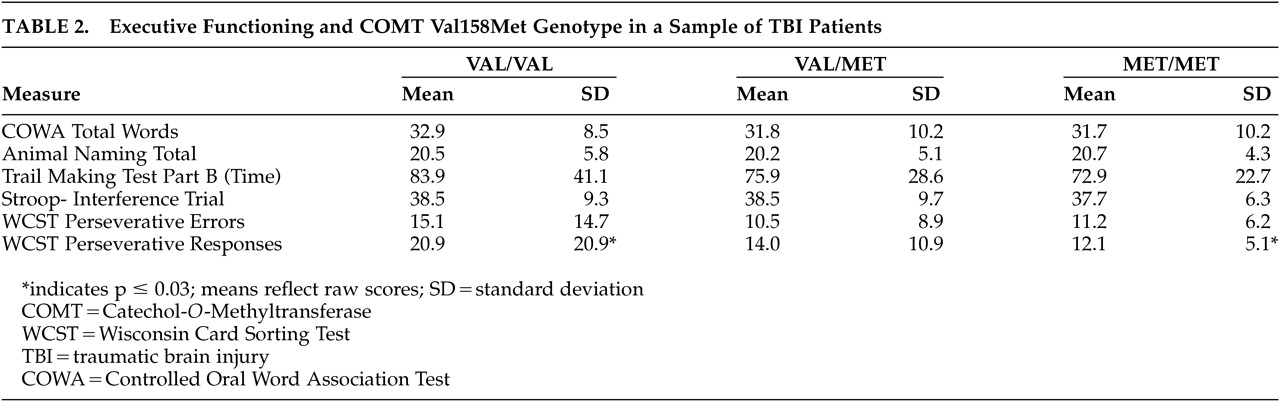
Analysis of variance with correction for multiple comparisons conducted on the executive functioning measures yielded a significant difference between genotype groups for WCST perseverative responses (p≤0.03) (
Table 2). WCST perseverative errors did not reach statistical significance. Posthoc examination of the perseverative responses revealed that the primary difference was between the homozygous groups, while the heterozygotes did not differ greatly from either homozygous group. Individuals homozygous for Met (Met/Met) made fewer perseverative responses on the WCST (Mean 12.1 [SD=5.1]) than did individuals homozygous for the Val variant (Val/Val), who had the highest number of perseverative responses (mean 20.0 [SD=20.9]). Heterozygotes made an intermediate number of perseverative responses (mean 14.0 [SD=10.9]). WCST perseverative errors did not reach statistical significance. No significant differences were found between the genotype groups with the tests administered thought to involve other aspects of executive functioning: animal naming, Stroop Color and Word Test, Controlled Oral Word Association, and time to complete Trail Making Test, Part B.
No genotype effect was seen in years of education completed or other demographic characteristics (
Table 1). As the study sample was predominantly Caucasian (N=71, 62.8%), this largest ethnic group was analyzed as a separate subpopulation. In this group, the Val to Met allele frequency was 0.50/0.50. The association of perseverative responses and genotype remained significant (p≤0.02), and perseverative errors tended toward significance (p≤0.054) with individuals homozygous for the Val variant (Val/Val) having a greater number of errors.
DISCUSSION
Our data demonstrate an association between COMT Val158Met genotype and an aspect of frontal-executive functioning following TBI. Specifically, COMT genotype showed an effect on perseveration as measured by the WCST. Perseverative responding suggests problems with cognitive flexibility in that the individual has difficulty shifting or changing mental set. This finding is generally consistent with the schizophrenia literature indicating greater perseveration on the WCST in schizophrenic patients with the Val/Val genotype.
9,12 In our study, only the perseverative responses variable on the WCST and not the perseverative errors variable was significantly different between groups. However, the homozygous Val group did show greater numbers of both perseverative responses and errors. The perseverative responses score from the WCST incorporates all types of perseverative responding including both errors and nonerrors. A perseverative response is a response that would have been correct in the preceding stage of the test and is thought to reflect difficulty shifting from one mental set to another. As the perseverative responses variable is a more inclusive score, it may have led to the more robust finding.
5,20 Moreover, Heaton, Grant, and Matthews
21 noted that the perseverative response score showed the highest sensitivity to cerebral disorders, especially disorders involving the frontal lobes, and as a result included only the perseverative response score in their comprehensive neuropsychological test normative project. The fact that none of the other tests tapping other aspects of executive functioning were significantly different between the genotypes is not surprising given the multifactorial nature of executive functions discussed earlier. Recent factor analytic studies of neuropsychological measures tapping frontal-executive abilities have demonstrated several different executive function domains
22,23 including cognitive flexibility, speed of processing, planning/initiation, and reasoning. In both of these studies, the WCST loaded onto factors reflecting cognitive flexibility whereas none of the other measures included in our battery loaded on this factor.
Taken together, these findings suggest that COMT, likely associated with levels of endogenous dopamine, may possibly influence certain deficits seen in frontal-executive performance after TBI. As mentioned above, questions remain as to whether the observed cognitive effects are related to the interaction of COMT genotype and TBI or would be observed by genotype alone. Some of the prior studies examining COMT genotype and WCST performance in schizophrenic patients and healthy comparison subjects have noted a similar effect of genotype on both groups.
9,11 However, comparison subjects with the higher enzyme activity Val allele did not perform as poorly as schizophrenic patients with the low enzyme activity Met allele
9,11 suggesting that genotype alone did not account for the findings. In fact, Egan et al.
9 determined that COMT genotype could account for approximately 4% of the variance in cognitive performance as evaluated by the WCST. Although we did not have the opportunity to evaluate healthy comparison subjects in this study, when one examines the performance of our TBI patients in relation to the normative sample of age and education similar peers,
20 the Val/Val group demonstrates below average performance for both perseverative responses (9th percentile) and perseverative errors (19th percentile). In contrast, both the Met/Met and Val/Met groups’ performance fell within the average range for perseverative responses (47th percentile; 39th percentile, respectively) and perseverative errors (47th percentile).
This finding of an association of COMT Val158 Met genotype and WCST performance following TBI is the second report of an association of a putative functional relationship of genotype and performance following TBI. Crawford and colleagues
24 reported that memory performance was worse in post-TBI patients with ApoE4 allele than in those with the E2 or E3 alleles.
Following TBI, it appears that low or moderate enzyme activity may result in less perseverative responding. As COMT genotype is likely associated with central dopamine levels, this interpretation is supported by the results of McDowell et al.,
25 who observed an effect of a dopamine receptor agonist, bromocriptine, on prefrontal function in TBI patients. Specifically, TBI patients given bromocriptine had significantly fewer WCST perseverative responses and improved performance on several other tests thought to involve executive function. Similarly, individuals with Parkinson’s disease have shown improvement on a number of neuropsychological measures during treatment with a selective inhibitor of COMT, Tolcapone.
26 Improved outcome may be functionally related to performance as determined in a functional MRI (fMRI) study, in which different COMT genotypes among well siblings from patients with schizophrenia were associated with differences in prefrontal cortex activity during performance of a different frontal-executive task involving working memory (visual
N-back task).
9 While the groups did not differ in terms of overall task performance accuracy, Val/Val individuals displayed greater activation (suggesting less efficiency) than Val/Met individuals, who displayed less efficiency than Met/Met individuals. These fMRI results suggest a link between prefrontal physiologic activity during performance of executive function tasks. This link could be mediated by changes in postsynaptic dopamine levels that may be influenced in part by the COMT Val158Met genotype.
Several limitations must be considered when weighing the findings of this study. First, the sample used is ethnically heterogeneous. The frequency of Met158/Met158 varies in different world populations. For instance, the frequency of Met158/Met158 in African populations is lower than in Caucasians,
8 as confirmed here. However, there was no significant difference between ethnic groups in perseverative responses. Furthermore, when the Caucasian subgroup was analyzed separately, the association between Met158 and a diminished number of perseverative responses remained statistically significant. Although this sample was primarily male, studies in healthy comparison subjects do not suggest a significant gender difference in the frequency of COMT Val158Met alleles. Therefore, sample gender bias should not affect the generalizations of these findings to samples with a more balanced gender distribution.
Another limitation is the possibility that multiple genetic factors could contribute to either poorer performance following TBI or protection that may enhance performance following injury. Another locus in linkage disequilibrium with COMT Val158Met may be responsible for the observed association with WCST performance. The COMT Val158Met polymorphism is certainly not the only contributing factor to recovery or cognitive performance status following TBI, although it is the only known functional polymorphism within the coding region of the COMT gene. It is plausible that promoter variants of the COMT gene are functional and would affect dopamine metabolism as well.
27 Future studies of the COMT gene promoter and other candidate functional variants from related genes would be justified, assuming larger study populations become available. At this time, we did not genotype any other gene markers, which would have reduced the power of this study to detect a functional effect of the COMT Val158Met polymorphism.
As this study was performed in a TBI population, the influence of other genes responding to trauma cannot be ruled out. This underscores the likelihood that behavioral outcome is multifactorial and that no single factor can account for complex behavior. Furthermore, the results presented here indicate only an association of COMT with executive function after TBI. The lack of a healthy comparison group limits the ability to examine a causative relationship between COMT genotype and executive performance following TBI. Therefore, one cannot determine from these data whether TBI played a role in the observed association between COMT Val158Met polymorphism and executive performance. However, this study extends the relationship between COMT genotype and executive function seen in healthy comparison subjects and individuals with schizophrenia to patients with TBI.
Future controlled studies are needed to elucidate the association between COMT and executive performance found in the present study. One small pilot sample of individuals with TBI and healthy comparison subjects found a similar association,
28 but larger sample sizes are needed to fully examine the putative role of COMT in TBI. Despite possible limitations, the data from this study extend the possible role of COMT in executive functioning found in healthy comparison subjects and schizophrenia to individuals with TBI.