Traumatic brain injury (TBI) frequently causes disturbances of the cerebrovascular circulation, which contribute to the increase of secondary damage.
1 Following severe TBI there may be reductions in cerebral blood fluid (CBF),
2 decreases in cerebral perfusion pressure (CPP), presence of vasospasm,
3 and impairment of autoregulation.
4Angiotensin-converting enzyme (ACE) is a component of the renin-angiotensin system (RAS), which plays an important role in BP regulation and electrolyte balance by converting angiotensin I to angiotensin II, a potent vasopressor, and by degrading bradykinin, a vasodilator. There is evidence of existence of an intracerebral RAS.
5 ACE is widely distributed throughout the brain and has been found to be 1) associated with the cerebral blood vessels, especially the choroid plexus; 2) associated with astrocytes of the periventricular nuclei; 3) in brain areas with high concentrations of angiotensin II receptors; and, paradoxically, 4) in areas where there are low concentrations of angiotensin II receptors, such as the basal ganglia.
6Previous studies have reported an association between the presence of at least one copy of the ACE D allele and greater risk of cerebrovascular disease
8 and cognitive impairment or dementia.
9 Experimental and human studies have demonstrated the beneficial effects of ACE inhibitor treatment on vascular injury,
10,11 hypertension,
12 brain ischemia,
13–15 and cognitive function.
11,12Since disturbances of the cerebrovascular circulation and ischemia are common in TBI and since experimental studies have suggested that reduced angiotensin II levels may decrease ischemic brain injury,
14,15 we hypothesized that ACE genes could be related to the neuropsychological sequelae in TBI survivors. The aim of the present study was to investigate whether cognitive functioning in the subacute phase differed in relation to the ACE I/D polymorphism in a sample of subjects with moderate and severe TBI.
RESULTS
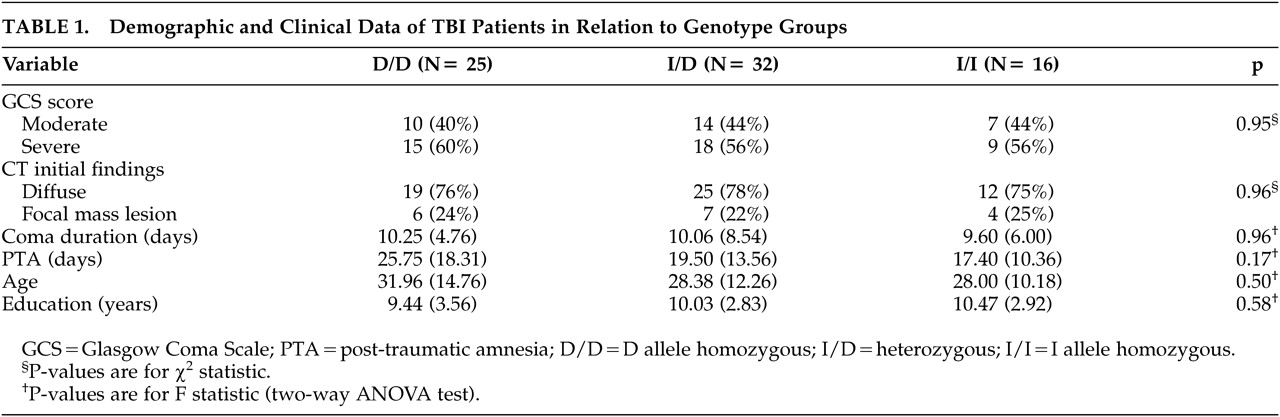
Demographic characteristics of the subgroups are displayed in
Table 1. Age, years of formal education, severity of injury, type of lesion according to the initial CT, duration of coma, and PTA did not differ among the TBI genetic subgroups. Genotype distributions for the TBI were in Hardy–Weinberg equilibrium (
Table 2).
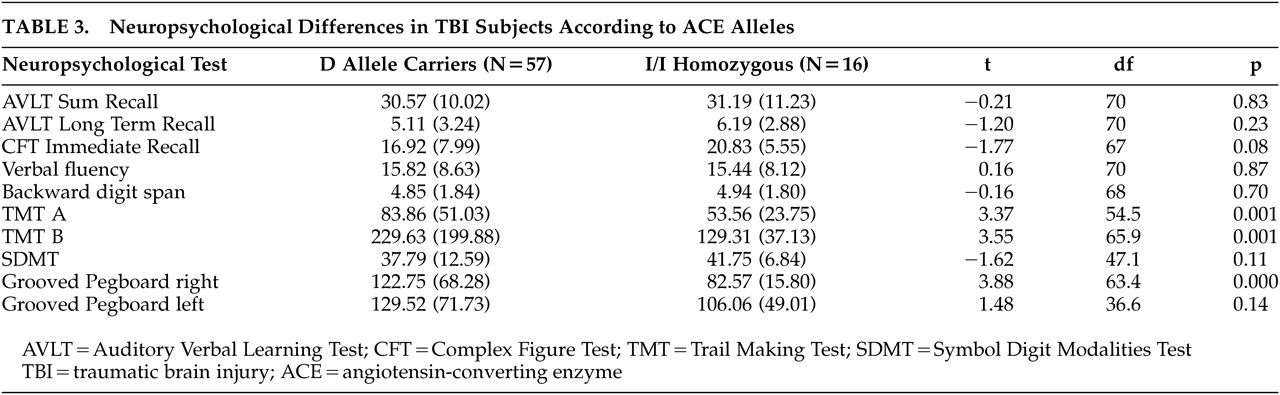
The ANOVA between the ACE subgroups revealed differences in Grooved Pegboard right hand (F=3.02, df=1, 65, p=0.05) and part A of the Trail Making Test (F=2.94, df=1, 72; p=0.06). Post hoc analysis (Tukey's honestly significant difference test) showed significantly poorer performance in both tests for the D/D than for the I/I genotype group (Grooved Pegboard right hand, p=0.043; part A of the Trail Making Test, p=0.050). When ACE I/I homozygous subjects (N=16) were further compared to ACE D allele carriers (N=57) (
Table 3), the performance of ACE D allele carrier patients was worse on the Trail Making Test Part A (t=3.37, df=54.5, p=0.001) and B (t=3.55, df=65.9, p=0.001) and Grooved Pegboard right (t=3.88, df=63.4, p=0.000).
DISCUSSION
To our knowledge, this study is the first to focus on the possible influence of the ACE I/D polymorphism on cognitive performance after TBI. In dementia and age-associated memory impairment the presence of the ACE D allele has been associated with poorer cognitive performance, especially on memory and frontal lobe functions.
21,22Our genetic subgroups did not differ in clinical variables that might cause differential neuropsychological impairment such as severity of injury, coma length, PTA duration or type of lesion according to the initial CT. Nor were there differences in other factors that might have influenced the prognosis of TBI such as age or years of education. We therefore attributed the differences in neuropsychological performance to the differences in ACE polymorphism. Our results showed poorer performance in D than in I/I carriers in frontal lobe functions involving visual scanning, fine motor speed, attention, and mental flexibility. Frontal lobe impairment in diffuse TBI has been related to white matter impairment,
23 and this impairment may be produced by vascular pathology. Cerebrovascular disturbances are common following TBI.
2,3 Decreases in global CBF or reduced regional CBF due to vasospasm are some of the sources of ischemia, one of the most frequent causes of brain tissue hypoxia in TBI. It has been hypothesized that cerebral ischemia may induce a change in local vascular receptor expression and function. It is important to note that a locally enhanced production of angiotensin II has been found after cerebral ischemia.
24 On the other hand, evidence from experimental studies has suggested that cerebral vasospasm after hemorrhage may be due to the vasoconstrictor effect of locally generated angiotensin II.
13Moreover, impairment or abolition of autoregulation is frequently observed after TBI.
4 Experimental evidence suggests a possible influence of ACE inhibitors on the blood pressure limits of autoregulation, protecting the brain against ischemia during sudden decreases in blood pressure
25 and that the selective blockade of the angiotensin II receptor type may present an important avenue in developing therapeutic strategies directed at prevention and alleviation of secondary brain injury after severe TBI.
26Since tissue and plasma ACE levels are higher in patients with the D allele, it is possible that the physiopathological changes associated with TBI may have greater consequences in patients with D/D or I/D genotypes than in those with I/I genotype.
Although this is an initial study, it could be replicated using a larger sample size. Additionally, an interrelational database of various genetic polymorphisms cross referenced to the degree of injury post TBI would be extremely useful.
Our results should be interpreted with caution in view of the numerous statistical comparisons, which raises the risk that some of the findings may be spurious. In addition, the small number of subjects of our cohort produces analyses with lower statistical power than some other studies. Since this is an exploratory study, we did not correct for multiple comparisons, mainly because the use of adjustments such as Bonferroni increases Type II errors.
27 We felt that with the low number of subjects the risk of inflating Type II errors was high enough already. The results reported here require confirmation in studies adequately powered to detect the effect of the ACE I/D polymorphism on neuropsychological outcome.
It should also be noted that our sample does not represent the full range of head-injured patients, since our subjects were selected with respect to their capacity for neuropsychological examination. It is possible that by selecting patients for our neuropsychological study from among the testable TBI survivors we may have introduced a bias that affected our findings. Therefore, our results can only be extrapolated to survivors of moderate-to-severe head injuries who recover to a testable level. Considering all head-injured survivors, it is likely that the cognitive impairments would be significantly greater.
On the other hand, the differences in neuropsychological outcome may certainly be related to differences in the location of lesions in the brain or the ultimate outcome of cerebral degeneration that may occur as a consequence of traumatic brain injury. Though our genetic groups did not show differences according to the initial CT findings, it is possible to argue that the CT scan in acute phase is a relatively poor predictor of outcome and may not be a good predictor of the development of subsequent lesions. However, in a recent report, our group showed a clear relationship between acute intracranial lesion diagnosis and neuropsychological results and ventricular dilatation indices at 6 months postinjury.
28 Additionally, cognitive sequelae may vary according to the type and localization of the mass lesion (epidural, subdural or intracerebral hematomas or brain contusion), thus it would have been useful to subdivide focal injury by localization of the injury, although we were unable to do so because of the small number of focal subjects in each genotype group. Further studies with larger samples and follow-up imaging data are required to control these factors.