Structural Studies
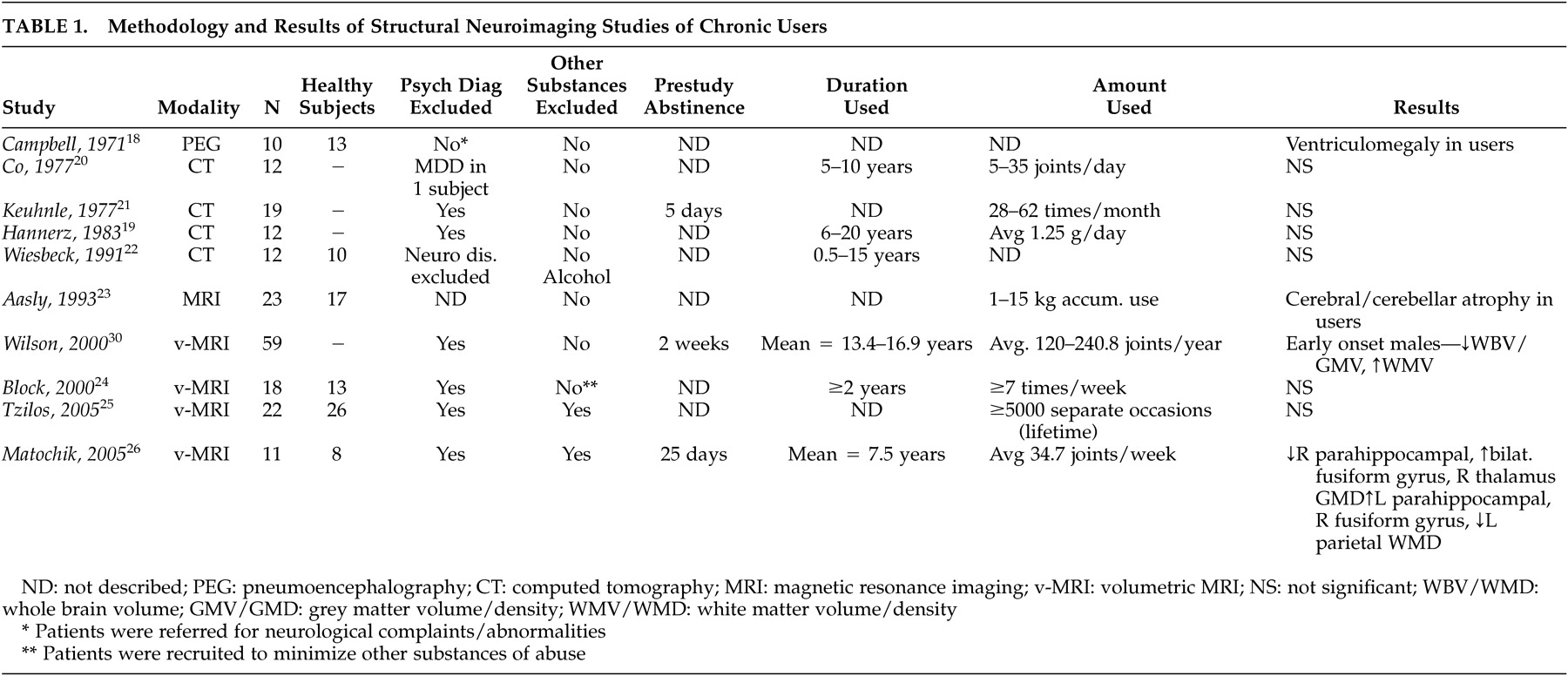
Ten structural imaging studies of chronic cannabis users have been published (
Table 1 ). An initial study using pneumoencephalography
18 suggested the presence of enlarged ventricles compared to neurologically healthy comparison subjects; however, multiple subject variables confounded the results, of particular relevance that patients were referred for heterogeneous neurological complaints. Subsequent studies employing CT
19 –
22 did not identify structural differences in chronic cannabis smokers, although limitations in study designs also existed and included a lack of comparison subjects, heavy comorbid substance use, and a wide variability in marijuana consumption.
Though one structural MRI study of cannabis users by Aasly et al.
23 reported abnormalities, volumetric measurements were not performed and the findings could not be attributed to cannabis use alone. Results were more consistent with the neurotoxic effects of alcohol (i.e., atrophy of the cerebellar vermis) as subjects all had significant alcohol (and other substance) use histories. Three methodologically rigorous volumetric MRI studies have been done to date with two reporting no significant differences between subjects and comparison subjects
24,
25 and one reporting significant differences.
26 The negative studies evaluated all brain regions, including analyses of regional and whole brain volumes, total white and gray matter, and hippocampal volumes. Though the earlier negative study
24 involved younger subjects (mean ages 22.3 and 22.6 years for men and women, respectively) where most subjects had used for less than 5 years, use histories were much longer in the more recent report.
25 The positive volumetric MRI study
26 reported chronic users compared to nonusing comparison subjects. Chronic users had significantly lower gray matter volumes in the right parahippocampal region while the corresponding left structure had higher white matter volumes. Differences were present in a number of other brain regions as well. Cannabis has been reported to have neurotoxic effects on the hippocampus in animal studies of brain pathology,
27,
28 but the evidence in human subjects is still lacking
29 and would not explain results differing between hemispheres unless cannabis exposure preferentially affected one hemisphere more than another.
In a study
30 of chronic cannabis users stratified by age of first use and gender, a significant correlation between age of first use and decreased total brain volume was seen. Unfortunately, nonusing subjects were not involved for comparison, so it is unclear if the findings represent differences from comparison subjects or whether they relate to other nonspecific factors that predict earlier onset of substance use and being at risk.
17 Later MRI studies
25,
26 did not find correlations based on age of onset in secondary analyses of imaging data.
In summary, minimal evidence has been found to suggest an association between cannabis use and structural brain abnormalities. Differences in subjects make comparisons between studies difficult, particularly with regards to amount and duration of cannabis used, as it could be hypothesized that toxicological effects may be more prominent only in heavy, long-standing users. Only one report using the highest resolution imaging technologies and analytic techniques available has identified differences, and it has not been replicated in other comparable studies, suggesting at this time that chronic cannabis use does not result in identifiable structural brain changes.
I. Cerebral Cortex
Global cortical effects have been found in functional imaging studies of chronic cannabis users with decreased activity during abstinence, and with generally increased activity with the administration of cannabis or THC, particularly in experienced users.
Initial studies utilized
131 Xe-inhalation scintigraphy (
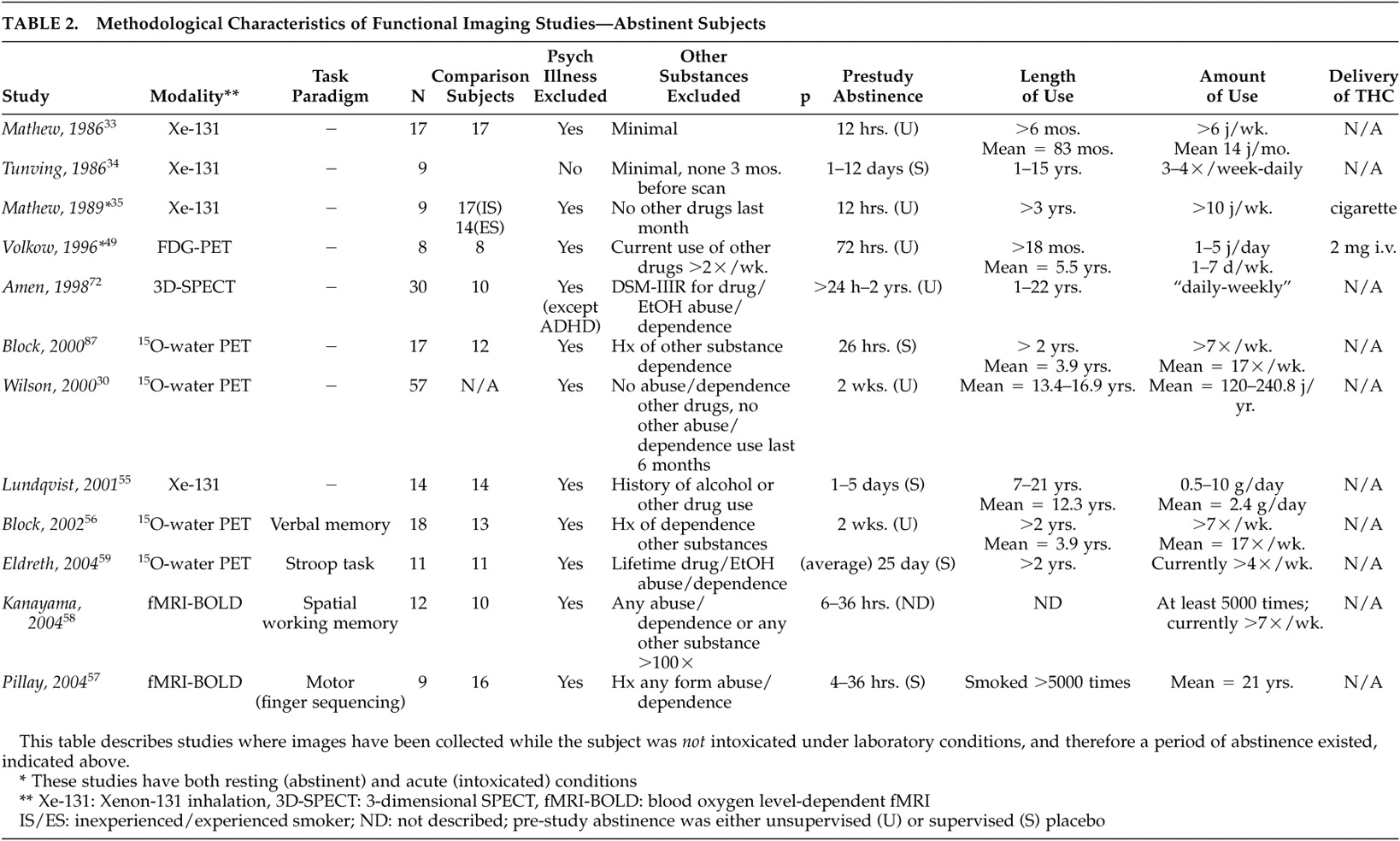
Tables 2 and
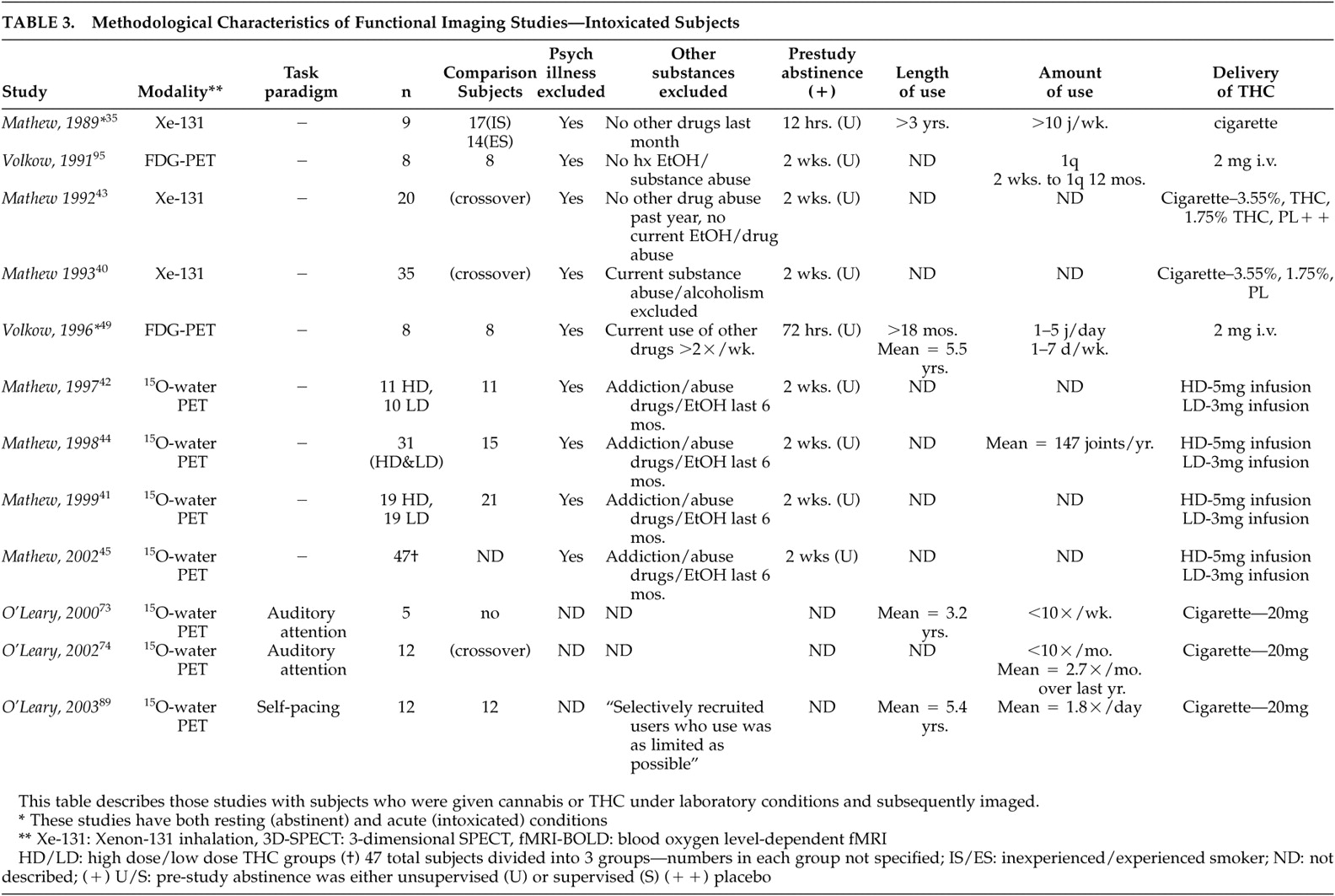
3 ), which is less sensitive to identifying subcortical or specific brain region activity changes than more modern functional imaging methods.
31,
32 The first report by Mathew et al.
33 compared abstinent, previously “heavy” cannabis users to individuals who had never used cannabis. There were no significant differences in cerebral blood flow (CBF) between the groups, although there was a trend toward decreased global CBF in cannabis users. In addition, this study suffers from the same problem that the majority of other functional imaging studies described herein, in that cannabis use (in this case “heavy”) is variably defined based on self-report with widely varying use patterns from subjects of varying backgrounds, making the comparison of study findings difficult. The second
131 Xe-inhalation scintigraphy study
34 examined nine subjects, in various stages of abstinence, who had been hospitalized for cannabis use problems and found significantly lower resting global brain CBF. The sample was not representative of habitual cannabis users, though, as subjects were recruited based on hospitalization without psychiatric comorbidity being excluded. Of interest, the study rescanned four subjects at various intervals 9 to 60 days after the first scan, reporting a 12% increase in CBF with one subject’s scan returning to baseline activity after 9 months of abstinence from cannabis. While rescanned subjects were not selected systematically, it suggests that the hypometabolism may have been a condition normalizing with abstinence. In a later study by Mathew et al.,
35 abstinent, “experienced” cannabis smokers exhibited significantly decreased resting global CBF compared to inexperienced cannabis smokers. “Inexperienced” cannabis smokers were required to be abstinent from cannabis for 3 years, but their use histories prior to that were not described.
In a continuation of their structural MRI study comparing early- and late-onset cannabis users, Wilson et al.
30 compared groups during abstinence using positron emission tomography (PET), further subdivided by gender. CBF was significantly greater in the male early-onset group compared to the male late-onset group. No significant differences were found, however, between the two female subgroups or between the early-onset men and women. Although it was postulated that alterations in cerebral volume and blood flow might be linked to cannabis inhibiting sex hormone production, affecting brain development in adolescence, a lack of healthy subjects made the conclusions highly speculative. Debate exists over the effect of age of first use: though some neurophysiological data have suggested that early use is a potential determinant of later brain function,
36,
37 neuropsychological data do not support this when accounting for verbal IQ.
7 Early-onset users may then simply represent a population at risk for multiple problems rather than cannabis use representing an etiologic basis for brain abnormalities.
17,
38The majority of studies have demonstrated global cortical activity increases during administration of smoked cannabis or infused THC. The 1989 study by Mathew et al.
35 examined subjects before and 60 minutes after smoking cannabis. Inexperienced users had a decrease in global cortical CBF while experienced users showed an increase. Anxiety scores were also significantly greater in the inexperienced group and negatively correlated with CBF. When compared with the placebo smoking session, inexperienced users were reported to have a significantly decreased global cortical CBF, but the finding may have just related to anxiety as no differences in CBF were seen in experienced users. The measurements of brain function, however, were taken at 60 minutes, longer than in most other studies discussed here, as well as exceeding likely times for peak neurophysiological activity for cannabis in the brain.
39Six studies by Mathew et al.
40 –
45 examined “occasional” users whose use histories were not well-defined. This group likely possessed different demographic, psychosocial, and neurobiological properties when compared with heavy using or better-defined groups, such as cannabis-dependent subjects. Despite this limitation, findings were fairly consistent. In two studies,
40,
43 increased global cortical activation was reported when comparing smoked (high- and low-dose) cannabis to placebo, where subjective intoxication correlated positively with global cortical CBF. Later studies
41,
42,
44,
45 using
15 O-water PET also found consistent increases in global cortical CBF after infused THC. In one study,
45 dose-dependent activation was found. Although the majority of the above studies have reported bilateral hemispheric activation during intoxication, some studies have reported significantly greater right hemispheric activation.
41,
43,
45 It has been theorized that differential right hemisphere activation may relate to cannabis’ purported tendency to promote creativity and increased emotional awareness.
45 –
47Two studies using
18 F-fluorodeoxyglucose (FDG)-PET to measure cerebral metabolic rate (CMR), however, have failed to demonstrate significant global cortical changes when subjects were administered intravenous THC. One study
48 reported variable changes in eight occasional users, with three subjects showing an increase, three a decrease, and two no change. A later study
49 using cannabis-dependent subjects compared with occasionally-using subjects did not show global cortical changes. It is not clear whether the absence of this finding in these two studies related to potential differences in users’ experience between smoked cannabis and intravenous THC, amounts of THC infused (both studies
48,
49 used a lower dose infusion of 2 mg versus 3 mg or 5 mg in the studies by Matthew et al.
41,
42,
44,
45 ) or to FDG-PET studying CMR rather than CBF. In general, however, the majority of studies, with the noted exceptions, have reported that smoked and infused cannabis result in increased global cortical activity, particularly in experienced users.
A. Frontal Lobes
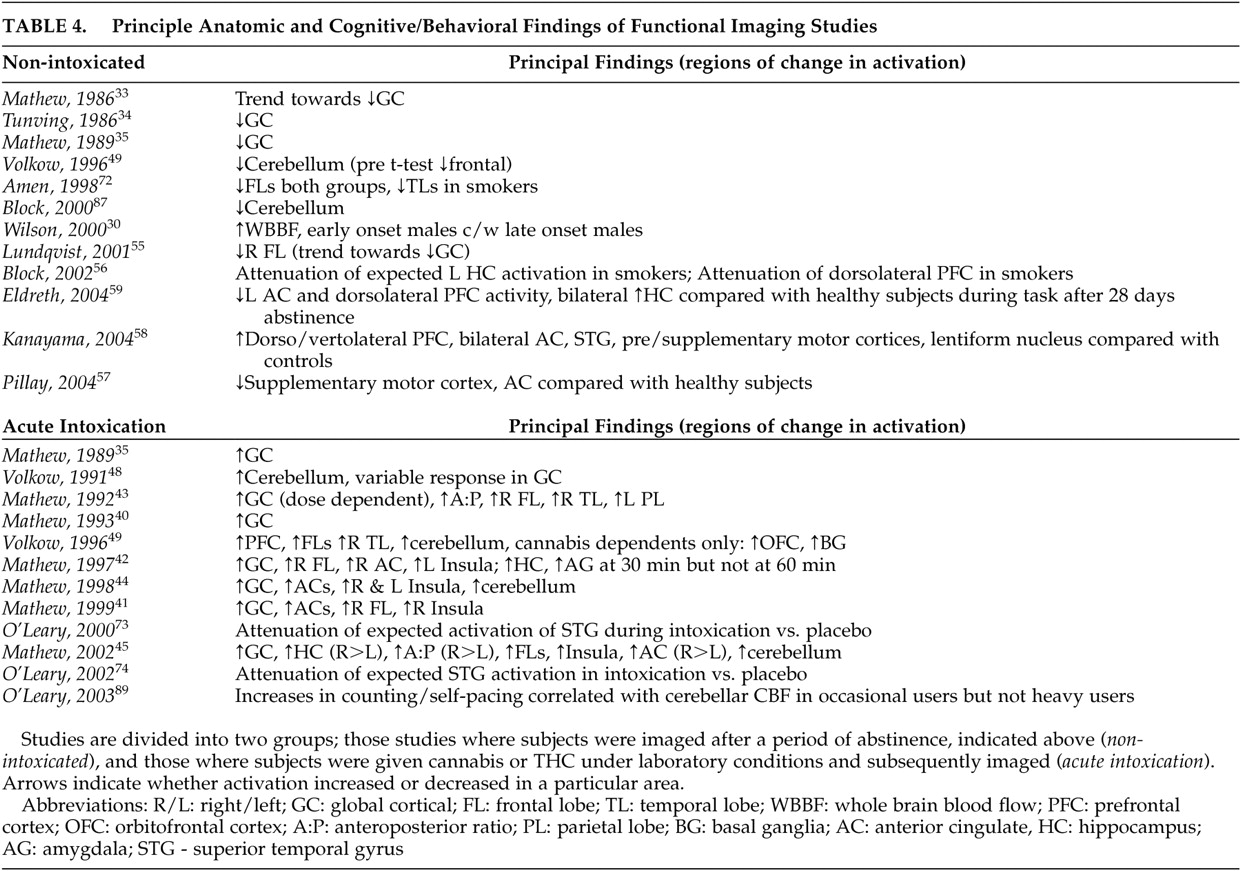
Like the global cortical changes reported, differential activity in specific lobes (particularly the frontal and temporal lobes) of the cerebral cortex has been reported, both with abstinence from chronic cannabis use and during the administration of smoked cannabis or infused THC (
Table 4 ). The frontal lobes are thought to play a significant role in substance use disorders
50 by their involvement in cognitive functions, including executive control, working memory, novelty processing, attention and awareness, and integration of multimodal sensory information.
51Chronic exposure to cannabis in rodents has been reported to result in decreased mesolimbic dopaminergic activity with associated reduced prefrontal cortex activity.
52 –
54 Lundqvist et al.
55 compared 14 abstinent DSM-IV cannabis-dependent subjects with 14 healthy subjects using
131 Xe-inhalation and found significantly reduced blood flow to the right frontal lobe. Their results differed from a similar study
34 which found only decreased global activity. The authors of the former study cited the improved resolution of their imager and shorter average time of abstinence prior to imaging as possible explanations. No correlations were found between flow and duration of use or total cannabis consumption. Volkow et al.
49 also noted decreased resting frontal lobe CMR via FDG-PET in abstinent cannabis-dependent subjects, but following post hoc t tests, this finding was no longer significant.
Mathew et al. have reported consistently increased right frontal lobe
42 –
44 and bilateral frontal lobe
40,
42,
45 activation following administration of smoked cannabis or infused THC in occasional cannabis users. While increased bilateral frontal lobe activity has been correlated with reports of subjective intoxication, temporal disintegration (the disrupted ability to subjectively perceive the passage of time) and depersonalization in some studies,
40,
42 others have reported right frontal activation more strongly correlating with subjective intoxication.
42,
44 Comparisons of anterior and posterior regional cortical changes in blood flow also support bilateral frontal lobe activation with smoked cannabis and infused THC correlating with reports of subjective intoxication.
43,
45 Volkow et al.
49 also reported increases in CMR 35 to 50 minutes post-THC infusion in the frontal lobes, including the prefrontal cortex. Both comparison and cannabis-dependent subjects demonstrated the increase, but only cannabis-dependent subjects had increased orbitofrontal cortex (and basal ganglia) activity.
To date there have been four task-based functional neuroimaging studies of recently abstinent regular cannabis users identifying differential frontal lobe activity.
56 –
59 Differences in task paradigms, sample characteristics, and imaging modalities appear to have led to differences in reported findings. Eldreth et al.
59 reported decreased bilateral dorsolateral prefrontal cortex (DLPFC) and right anterior ventromedial prefrontal cortex (VMPFC) activity with a modified Stroop test (a test requiring subjects to ignore distractors during target detection; it has been shown to activate medial and lateral prefrontal cortices and the anterior cingulate in healthy subjects)
60,
61 in heavy users abstinent for 25 days compared to healthy subjects. No differences were seen in task performance between the two groups, who were matched for IQ and years of education, among other characteristics. The authors postulated that exposure to chronic, heavy cannabis use may result in the activation of alternate neural pathways to process information and thus maintain task performance. Block et al.
56 reported attenuated left DLPFC and left hippocampal activity with a verbal memory task in heavy users abstinent for 2 weeks compared to healthy subjects. Cannabis users had more difficulty with the task, although IQ was not measured in either group, nor was educational background. Their finding parallels neuropsychological testing results in which verbal recall was reported as impaired in heavy users at 7 days, but returned to normal at 28 days.
7 Pillay et al.
57 reported decreased anterior cingulate and supplementary motor cortex (SMC) activity with a finger sequencing task used to examine fine motor function in abstinent heavy users compared to healthy subjects. Although subjects had a brief and somewhat variable (4- to 36-hour) period of abstinence, quantified urinary cannabinoid metabolites did not correlate with task performance, nor was performance correlated with IQ. Subject and comparison groups were not compared for IQ, but there was no difference in years of education. In contrast, one study
58 reported increased bilateral DLPFC and ventrolateral prefrontal cortex (VLPFC) activity with a visuospatial memory task in abstinent cannabis users compared to healthy subjects. The differences found in this last study may have related to a slightly longer period of abstinence prior to testing and potential compensatory changes. No correlation to task performance with urinary cannabinoid metabolites or IQ was seen within the subject group, but the latter variable was not measured in comparison subjects. Years of education were comparable between groups. While the report by Block et al.
56 demonstrated definitive differences in task performance, they were not apparent in the other three, despite differences in regional activation. The task-based studies, then, by finding changes in comparison to healthy subjects in expected areas of activation, suggest that chronic cannabis use may attenuate or at least alter cerebral functions responsible for higher level cognitive processing, which persists at least during initial abstinence.
In summary, functional neuroimaging studies have reported generally consistent frontal lobe differences. Attenuated frontal lobe activity has been reported in recently abstinent heavy cannabis users compared to healthy subjects in resting and task-based studies, while increased frontal lobe activity has been generally reported with cannabis exposure in both heavy and occasional users, correlating with subjective reports of intoxication. The endocannabinoid system is believed to be functionally linked to the extended dopamine reward pathway involving the ventral tegmental area, nucleus accumbens, prefrontal cortex (including the orbitofrontal and DLPFC), and anterior cingulate,
62,
63 viewed as central to the development of addictive behavior.
64 In rats, administration of THC is reported to increase firing of dopaminergic neurons in the ventral tegmental area.
65 –
68 Differential activity to substance-related cues in the prefrontal cortex has been consistently reported in substance-dependent subjects.
50,
69,
70 Changes in the reported frontal lobe activity from neuroimaging studies to date have been consistent with those found with other substance use disorders.
B. Temporal Lobes
The cortical areas of the temporal lobes are related to audition, speech, and integration of sensory information.
71 The only study to report findings during abstinence was by Amen and Waugh,
72 who used three-dimensional SPECT, and reported decreased temporal lobe activity in heavy cannabis users with comorbid attention deficit hyperactivity disorder (ADHD) compared with subjects with ADHD alone, but who were variably bilateral, right-, or left-sided. The psychiatric comorbidity may have confounded the findings, as both diagnoses may have specific interactional effects as opposed to simply additive ones. Subject differences were also marked regarding durations of abstinence (ranging from 24 hours to 2 years) and duration of use.
In two studies, Mathew et al.
40,
43 reported right temporal lobe activation with smoked cannabis in regular cannabis users which, in one study,
40 correlated with subjective reports of intoxication, depersonalization, and temporal disintegration. Right temporal lobe increases in CMR were also reported in an FDG-PET study
49 in both dependent and occasional users.
Two PET studies by O’Leary et al.
73,
74 evaluated cannabis users via a dichotic listening task to evaluate auditory attention when administered smoked cannabis. This task has previously been reported to result in activation of the right superior temporal gyrus in healthy, nonexposed subjects.
75 The first was a pilot study of five subjects and lacked a comparison group. Although no differences in task performance were identified between cannabis and placebo administered groups, those who smoked cannabis showed attenuated superior temporal gyrus CBF. Other regions in frontal and parietal areas also showed lower CBF compared with placebo. The decreased activity reported stands in contrast to the pattern of increased global and frontal activity described above. O’Leary et al. concluded that cannabis intoxication may inhibit structures forming an attentional network, but intelligence or education background was not described in either report.
Overall, results of functional neuroimaging studies identifying the temporal lobes are limited and somewhat conflicting. They suggest increased activity with intoxication, correlating to subjective effects in regular cannabis users, but attenuation of activity in tasks requiring attention. Any conclusions would be speculative at this point.
III. Cerebellum
There is mounting evidence for cerebellar involvement in higher cognition, including executive function, memory, language, timing, as well as emotional processing.
85,
86 Differential cerebellar activity has been reported in several functional neuroimaging studies with chronic cannabis users and acute exposure to cannabis (
Table 4 ).
Earlier studies using SPECT generally would not show changes in cerebellar metabolism as this technique does not visualize subcortical structures well.
32 Volkow et al.
49 compared eight abstinent cannabis-dependent subjects to eight healthy subjects via FDG-PET measuring CMR and reported cerebellar hypometabolism in the cannabis-dependent subjects compared to the healthy subjects. No other regional changes were seen. Greatly reduced cerebellar CBF was also reported in a well-designed volumetric MRI study by Block et al.
87 using
15 O-water PET to compare 17 abstinent regular cannabis users with 12 non-using healthy subjects. Decreased cerebellar metabolism has been demonstrated following chronic in vivo administration of THC to rodents.
88 This effect is thought to be secondary to impaired spontaneous network activity of cerebellar granule cells, suggesting a direct mechanism of action on cerebellar dysfunction, in addition to indirect phenomena, discussed below.
With the administration of smoked cannabis or infused THC, increases in cerebellar activity have been reported in occasional and regular cannabis smokers (
Table 4 ). Mathew et al.
44 reported significant (although variable in individual subjects) increases in cerebellar blood flow in one study and a trend toward increased cerebellar blood flow (not significant) in another.
42 The former study also reported a significant correlation of pontine CBF to cerebellar blood flow, leading the authors to suggest that the findings related to a frontocerebellar network in which the pons is involved.
44 A later study by the same authors
45 found cerebellar activation to be present up to 60 minutes after smoking cannabis, but it was not significant compared with placebo at 90 minutes. Volkow et al.
48,
49 also reported significant increases in cerebellar activity with infused THC in both occasional cannabis users
48 and cannabis-dependent subjects
49 compared to healthy subjects. Subjective reports of intoxication were positively correlated with cerebellar blood flow,
48,
49 but inversely correlated with the subjective reporting of temporal disintegration.
44In a task-based study, O’Leary et al.
89 evaluated neural correlates of self-pacing during acute intoxication with smoked cannabis via
15 O-water PET in occasional and heavy cannabis smokers compared with healthy subjects. No differences were present in IQ or education. Subjects were scanned twice (1 week apart), while counting verbally at a predetermined, practiced rate before and after smoking a cannabis cigarette in one session and a placebo in the other. Counting rate increased in both groups after smoking cannabis but did not change after placebo. Following cannabis, counting rate negatively correlated with left inferior posterior cerebellar metabolism in the occasional cannabis smokers, although this was not present in the heavy cannabis-smoking group (a trend toward negative correlation using a lower statistical threshold was found for the anterior vermis in this group).
In general, cannabis-dependent and regular cannabis-using subjects during abstinence have been found to show reduced cerebellar activity, but with cannabis exposure, both groups show increased cerebellar activity correlating with subjective reports of intoxication. Temporal disintegration, however, has been reported to be inversely correlated with cerebellar activity, while increased metabolism in this region during acute intoxication alters self-pacing, another timing function. These data are consistent with the hypothesis that the cerebellum is highly involved in the subjective experience of time passage as well as self-pacing, and that these functions are disrupted during acute cannabis intoxication with persistence during (early) abstinence.