T he differential diagnosis of patients who experience symptoms of paresthesias, derealization, dizziness, chest pain, tremors, and palpitations can be quite challenging. These symptoms occur across a wide range of disorders, including cardiac, psychiatric, and neurological disorders. It can be particularly difficult to differentiate partial seizures without generalization of temporal lobe origin from panic disorder, as all of the above symptoms can be found in both conditions.
7,
8 In fact, panic disorder has been found to be the most common condition that must be distinguished from seizure disorder.
9 The possibility that panic disorder and temporal lobe epilepsy with ictal fear can be comorbid has also been raised.
10 Panic disorder occurs in 1% to 3% of the population. Older estimates place the incidence of seizures at 0.03%, with ictal fear occurring in one-third of these patients.
11,
12 New estimates place lifetime incidence of epilepsy at 3%.
13 Although a rare occurrence, multiple case reports have documented that patients initially diagnosed with panic disorder may later receive a diagnosis of temporal lobe seizures. Initial patient presentation can be quite varied.
14 –
18 It has been proposed that panic attacks with an onset consistent with an epileptic aura may sometimes be the result of simple partial seizures with a psychological presentation.
19 This hypothesis is supported by several lines of evidence, including concomitant symptoms, multiple cases with initial diagnosis of panic disorder but eventual electroencephalographic (EEG) documentation of seizures, comorbidity of the two conditions, nonepileptic EEG abnormalities in panic disorder, the proposed amygdala-driven kindling of the fear network, and limited clinical data suggesting successful treatment of panic attacks with antiepileptic medications.
19The literature does provide guidance for distinguishing between these two conditions:
8,
11 panic attacks are generally longer in duration than seizures; ictal episodes are more stereotyped, whereas panic episodes are likely to vary more in presentation; although seizure disorders may initially present with fear/anxiety, they may progress to the more classic symptoms (e.g., olfactory auras, aphasias, amnestic features, motionless state, visceral automatisms); initial presentation of these classic epileptic features (i.e., before the fear/panic feelings) suggests seizures; panic disorder is more likely associated with agoraphobia (50% of cases); panic disorder has stronger familial links (25% for first-degree relatives); panic disorder can be worsened by emotional distress; presence of temporal lobe lesions on imaging can indicate epilepsy; and some treatments for panic disorder can worsen seizures (e.g., tricyclic antidepressants).
8,
9 If the presentation is suggestive of epilepsy, EEG examination may be required to identify the characteristic spike/wave pattern of seizure discharge. A full evaluation for epilepsy may require 24-hour EEG and video monitoring or intracerebral depth electrodes with subdural grid arrays, as these procedures can sometimes identify abnormal electrical discharges not observed on routine EEG.
18,
20,
21 High resolution magnetic resonance imaging (MRI) is recommended for visualization of the deep temporal lobe structures.
2,
22 This is particularly important for reliable separation of the amygdala and hippocampus in order to obtain accurate volume measurements.
23 Given the similar clinical features and divergent treatments for these two diagnoses, it is imperative for the clinician to understand the neuroanatomical features of the temporal lobe and the noninvasive imaging techniques available to assist in decision-making.
Anatomy of the Temporal Lobe
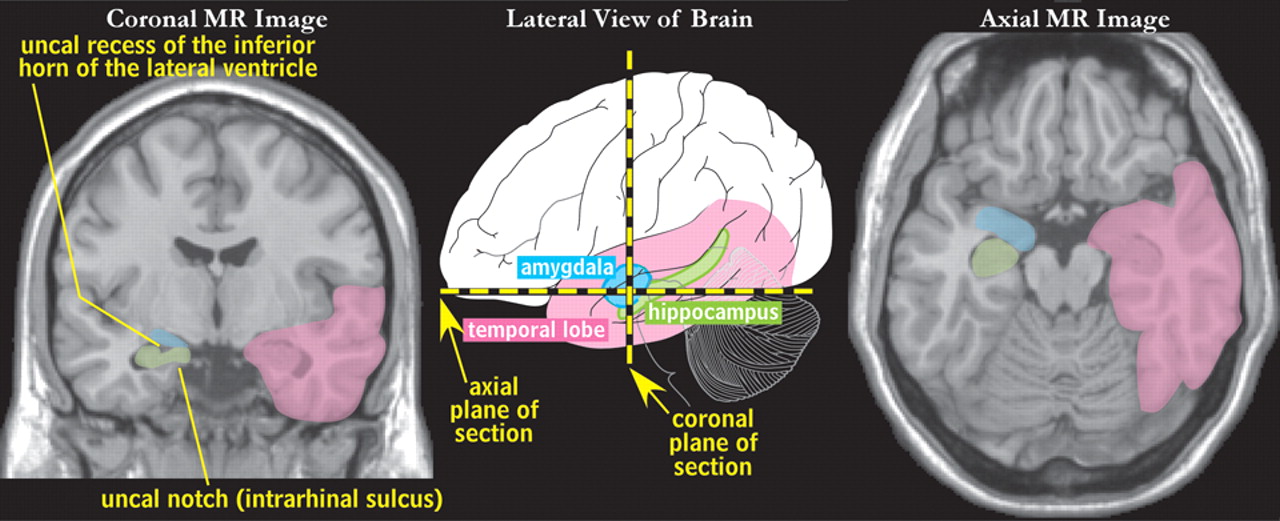
The mesial temporal lobe contains structures that are part of the limbic circuits, including the amygdala and hippocampus (
Figure 1 ).
1,
2,
24 These structures have been implicated in the genesis of both panic disorder and seizure disorders.
8,
9,
18 Fear and panic have been elicited by electrical stimulation of the amygdala.
25 –
29 These symptoms can also occur as the initial symptoms of temporal lobe discharges.
7,
14,
30 Abnormal electrical activity in the frontal lobes, due either to spread from the temporal lobe or to a frontal focus, has also been found to be associated with fear or panic.
30Structural Imaging
Temporal Lobe Epilepsy
It is a common finding that temporal lobe structures, particularly the hippocampus and amygdala, are decreased in volume in patients with temporal lobe epilepsy.
23,
31 One study reported that amygdala volume was more decreased in temporal lobe epilepsy patients who reported experiencing fear during seizure onset than in those who did not.
32 However, a later study from the same group did not confirm this association.
33Panic Disorder
Earlier studies compared volumetric measurement of temporal lobe structures between patients with panic disorder and healthy individuals based upon edge-tracing regions-of-interest.
34 –
36 One study reported that only the amygdala was decreased in size (bilaterally).
34 This study found no differences in the volumes of the hippocampus or the whole temporal lobe, and no anatomic measure correlated significantly with any clinical or demographic measure. In contrast, two studies found the volume of the temporal lobe (on the left in one, bilaterally in the other) was significantly decreased, but not the volume of the hippocampus or amygdala.
35,
36 One study found a perplexing inverse correlation between duration of panic disorder and hippocampal volume, with a more recent onset associated with a smaller hippocampus.
35 Recently, several groups have utilized voxel-based morphometry to compare gray matter volumes of patients with panic disorder (diagnosis confirmed by a structured clinical interview [SCID], no mention made of any EEG studies) to healthy individuals.
37 –
39 With strict statistical criteria applied, there is little agreement, with one study reporting decreased gray matter only in the parahippocampal gyrus, the second reporting decreases only in the putamen, and the third reporting increased gray matter in several areas of the brainstem as well as ventral hippocampus. With less stringent statistical criteria, decreased gray matter volume was also found in the inferior and superior frontal and temporal gyri, and increased gray matter volume was found in the middle temporal gyrus in at least two of the three studies, although the laterality was not always the same. None reported changes in the amygdala. An inverse correlation was reported between volume of the putamen and clinical symptoms, with a lower volume associated with greater symptom severity and illness duration.
38The diversity of these findings may be due, at least in part, to differences in the measurement techniques employed. Edge-tracing region-of-interest analysis is based on the recognition of anatomic landmarks that are used to hand-trace regions onto magnetic resonance images. This approach has the advantage that individual differences in anatomy can be easily taken into account. It is also stronger statistically, because fewer comparisons need to be made. However, fewer areas can be assessed. Anatomic criteria and anatomic expertise vary across studies. Image resolution and section thickness also vary, a critical factor when small structures, such as the amygdala, are measured. Voxel-based morphometry provides a method of automatic analysis of the entire brain, allowing many more areas to be compared. However, this creates a statistical challenge. The likelihood of getting a false positive increases with the number of comparisons made. A mathematical correction for multiple comparisons must be performed. This approach also requires transformation of each individual’s data onto an average brain template, which inevitably results in some distortion of individual anatomy, and often in some loss in image resolution, particularly if smoothing is also performed. In addition, image voxels are relatively large in terms of the size of many structures in the brain. If 1-mm sections with an in-plane resolution of 1-mm are acquired, for example, the cortical ribbon would be no more than three voxels wide. As a result, many voxels contain both gray and white matter, and partial-volume averaging is inevitable.
Finally, there is also diversity across studies including patient population and medication status. It should also be noted that EEG was not used to rule out any electrical abnormalities. As noted above, temporal lobe epilepsy has also been associated with reduced amygdala volumes as well as multiple areas of temporal lobe injuries.
Functional Imaging—Cerebral Blood Flow/Cerebral Metabolism
Given the common abnormal structural findings in both conditions, functional imaging may be needed to further inform the differential for panic disorder from temporal lobe epilepsy.
Temporal Lobe Epilepsy
During an epileptic seizure (ictus) there is an increase in both blood flow and metabolism in the seizure focus. Generally, seizures are brief, lasting only seconds to minutes. During the immediate postictal period, there is a drastic fall to a state of severe hypoperfusion. This is followed by a gradual recovery to a lessened level of hypoperfusion, which becomes the new resting state. This sequence is called the “postictal switch.” Thus, interictally, there is a general hypometabolism in the abnormal area, and at times in the surrounding cortex, subcortical nuclei, or even in the contralateral temporal lobe. Comparative studies indicate that this depressed state deepens with the duration of the seizure disorder, with cerebral metabolism more affected than cerebral blood flow.
40 This mismatch between metabolism and blood flow indicates that 18-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET), which provides images of cerebral metabolic rate, will more accurately delineate the seizure focus for scans acquired during the interictal period than single photon emission tomography (SPECT), which provides images of cerebral blood flow.
40,
41Both SPECT and FDG-PET are used to identify areas of seizure focus, evaluate patients for surgical resection of the affected temporal cortex, and to predict clinical outcome postsurgery.
13,
31 A major advantage of SPECT is the ability to capture ictal activity (i.e., hyperperfusion), due to rapid (e.g., 30 to 60 seconds) radiotracer uptake.
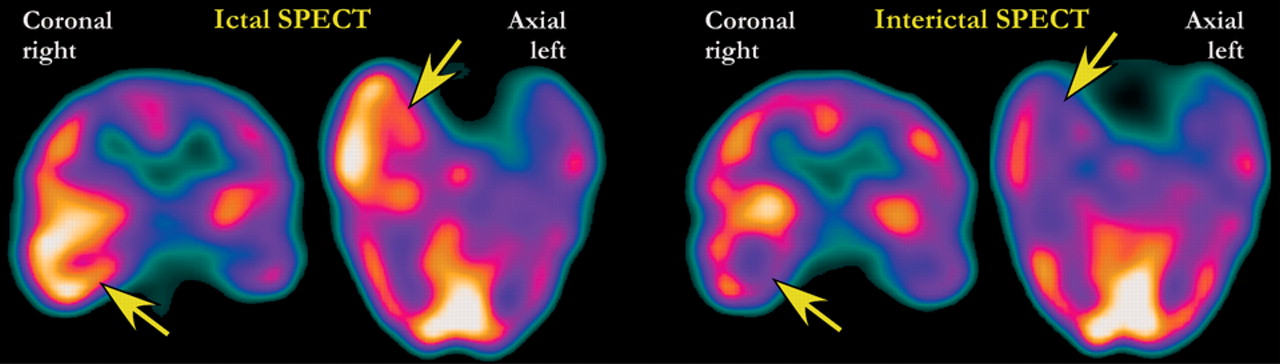
31 Ictal SPECT scans are compared to the interictal SPECT to determine the area of seizure focus (areas that are “hot” during the seizure and “cold” between seizures) (
Figure 2 ). The sensitivity and accuracy of this technique are reported to be approximately 80%.
13 In clinical practice it is likely to be somewhat lower than this. Many research studies include patients for whom nuclear imaging would not be required because the seizure focus can be identified by visualization of lesions on structural images.
FDG-PET has higher image resolution, but radiotracer uptake is generally too slow for ictal imaging. The better spatial resolution of interictal FDG-PET scanning does allow for quantitative evaluation of abnormal areas that can be compared with the structural magnetic resonance (MR) images. Current FDG-PET techniques allow statistical parametric mapping that can identify abnormal areas sometimes not evident on visual interpretation. Both FDG-PET and SPECT are more valuable in conjunction with inpatient video EEG monitoring. Partial-volume averaging may cause small areas of abnormality to artificially appear healthier than they really are. Nonlimbic (nontemporal lobe) seizures are more likely to have normal interictal scans than are limbic/temporal lesions. FDG-PET and SPECT may identify patients who have two independent areas of seizure focus, not identifiable on structural imaging or by EEG data. Additionally, they can identify temporal lobe dysfunction in atypical panic/fear episodes signaling the need for an epilepsy investigation. Two recent case reports illustrate a clinical presentation of atypical fear/panic attacks resulting from mesial temporal sclerosis. In each case, nuclear imaging identified the temporal lobe hypometabolism/hypoperfusion of the epileptic focus.
17,
42Areas of abnormal metabolism or blood flow may be present at a distance from the epileptic focus. Statistical parametric mapping was used to assess the extent of both ictal and interictal cerebral blood flow (SPECT) alterations in patients with mesial temporal sclerosis compared with healthy individuals.
43 In addition to the expected increased perfusion in the ipsilateral temporal lobe (including the temporal stem white matter), ictal SPECT identified areas of hyperperfusion in the contralateral temporal lobe, anterior frontal lobe (left-sided focus) and parietal lobe (right-sided focus). Areas of decreased perfusion on interictal SPECT included hippocampus, thalamus, midbrain, superior paracentral lobule, insula (left-sided focus), and cingulate gyrus (right-sided focus). The authors of this study noted that these results are consistent with functional impairment of the cortico-thalamo-hippocampal circuit. A recent study compared the localization of interictal hypometabolism (FDG-PET) with the outcome of surgery.
44 Patients with hypometabolism confined to the temporal cortex containing the focus had better outcomes (78% seizure-free) than patients with hypometabolism in additional ipsilateral areas (45% seizure-free) or contralateral areas (22% seizure-free).
Panic Disorder
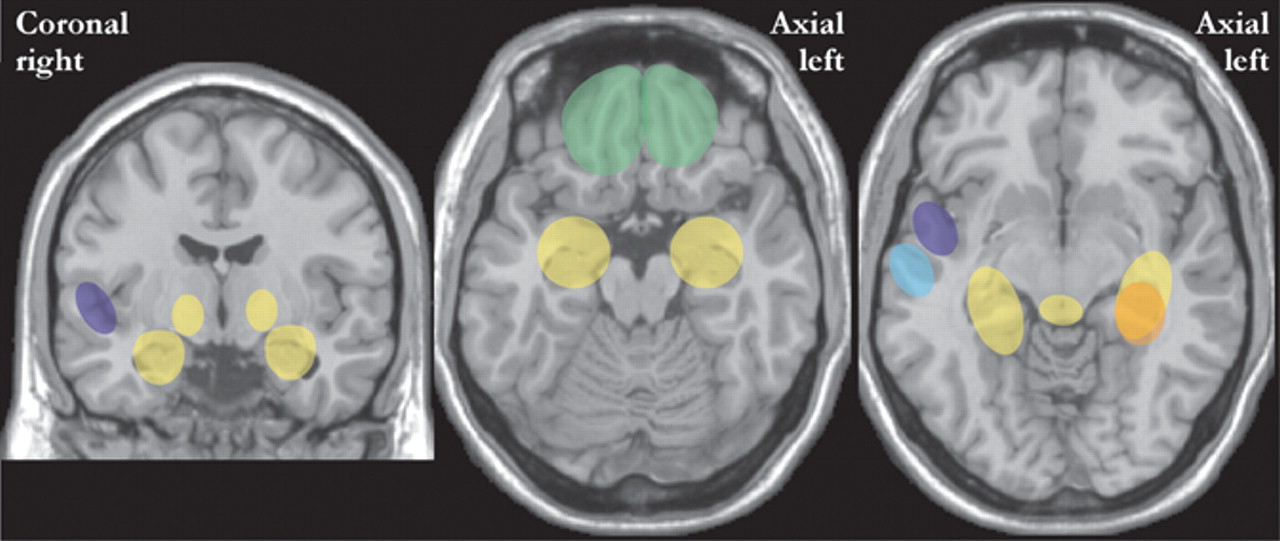
Two studies have used statistical parametric mapping to compare cerebral metabolic rate (FDG-PET) of patients with panic disorder (medication free) with healthy individuals (
Figure 3 ).
4,
6 Both found increases in hippocampus and parahippocampal structures. One also found increases in the thalamus, cerebellum, medulla, and pons.
4 The authors of this study
4 noted that these areas are part of the amygdala-based fear network. The other study found decreases in inferior parietal and superior temporal areas.
6 A third study of medication-free patients with panic disorder utilized visual and semiquantitative analysis of regional cerebral blood flow (SPECT).
5 They found significantly decreased perfusion only in the inferior frontal area (
Figure 3 ). The authors commented that this might be due to an inhibitory influence from the amygdala. None of the above studies reported any correlation between functional imaging findings and clinical symptoms. In contrast, a study that compared cerebral perfusion (SPECT) in medicated patients with panic disorder to healthy individuals found decreases only in the superior temporal lobe (
Figure 3, right ). This study reported an inverse correlation between blood flow and both duration of illness and clinical symptoms (lower perfusion with higher scores).
3 One group performed a second functional imaging examination (FDG-PET) at the conclusion of 10 sessions (over 6 months) of cognitive behavior therapy.
45 Most of the patients (11/12) were responsive to treatment. In comparison with pre-treatment levels, the responsive group exhibited normalization of cerebral metabolism, with decreases in the hippocampus, cerebellum, and pons, and increases in the medial prefrontal cortex bilaterally. Overall, these results are consistent with altered reactivity in the fear circuitry that may normalize with successful treatment.
Functional Imaging—Receptor/Neurotransmitter Mapping
As radio-tracers for a variety of neurotransmitter receptors become more widely available, they add yet another nuclear imaging tool that may help to clarify temporal lobe function and pathology. At the present time, ligands for the benzodiazepine-GABA A receptor, the serotonin 5-HT 1A receptor, and the muscarinic acetylcholine receptor have been evaluated for their ability to improve visualization of the epileptic focus. Of these, only a handful of studies utilizing some of these ligands have been performed in patients with panic disorder.
Benzodiazepine-GABA A Receptor
GABA has been known to be associated with the temporal lobe, anxiety disorders, and seizures for many years.
46,
47 Ligands for the central benzodiazepine-GABA
A receptor suitable for both PET (C
11 flumazenil, FMZ) and SPECT (I
123 iomazenil, IMZ) studies are available, although neither is yet approved for clinical use in the United States.
48Temporal Lobe Epilepsy
Reduced benzodiazepine receptor binding has been demonstrated (by autoradiography) in surgically resected specimens from temporal lobe epilepsy patients.
49 It has not yet been proven that either of the available benzodiazepine ligands is more accurate for delineation of the seizure focus than the functional imaging techniques already in clinical use (as detailed above).
48,
50 –
52 There are promising indications. When hippocampal sclerosis was present, the area of abnormality was often larger on FDG-PET than on FMZ-PET, which more closely matched the epileptogenic zone as determined by intracranial EEG.
50 A case was recently published in which interictal IMZ SPECT provided correct laterality of the seizure focus (as determined by intracranial EEG) while ictal perfusion SPECT did not.
53 Timing of the examination in relation to seizure activity may be a critical factor, as a recent study found within-subject short-term changes in binding.
54 Lower values (better delineation of seizure focus) were obtained when the FMZ-PET was performed shortly after a seizure, suggesting a transient decrease in expression or availability. Given this evidence for dynamic seizure-related changes, the authors of the study recommend that benzodiazepine receptor imaging be performed within a few days following a seizure for best results. Larger studies in which this factor is taken into account are needed to better assess the value of this technique. Other potentially confounding factors include medication status (many antiepileptic medications affect GABA), comorbid psychiatric diagnoses, and variations in imaging technology.
Panic Disorder
Both increases and decreases in regional benzodiazepine binding have been reported when patients with panic disorder were compared with healthy individuals.
55 –
57 Two studies included only patients with panic disorder that had never taken benzodiazepines.
55,
56 One, using FMZ-PET and statistical parametric mapping, reported both globally reduced binding and regional decreases in orbital (right) and insular (right) cortices in patients compared with healthy individuals.
55 The other, using IMZ-SPECT and template-based analysis, reported increased binding in orbital cortex (right) and a trend toward increased binding in temporal cortex (right) in patients with panic disorder.
56 The third study included patients with panic disorder who had been medication-free for at least 6 weeks.
58 This IMZ-SPECT study utilized both statistical parametric mapping and region-of-interest analyses. No difference was found in global binding between the patient group and healthy individuals. Binding was decreased in the area of the hippocampus (left) and precuneus (left) and increased in caudate (right), medial frontal cortex (right) and middle temporal gyrus (left) in the patient group. Some patients experienced panic symptoms during scanning. Decreased binding in the medial frontal and superior frontal cortex (Brodmann’s areas 8, 9, 10) correlated with increased symptoms. Diversity across studies may be due to methodological differences and/or variations in the patient population studied. While preliminary, these studies suggest that alterations in benzodiazepine binding in frontal areas may differentiate panic disorder patients from temporal lobe epilepsy patients. Further studies that explore the apparently dynamic relationship between symptom state and binding would be of great value.
Serotonin 5-HT 1A Receptor
Serotonin has been implicated in the pathophysiology of both panic disorder and seizure disorders.
59,
60 Ligands based on several 5-HT
1A antagonists have been developed for PET imaging (C
11 WAY100635; F
18 trans-4 - fluoro - N - 2 - [4 - (2 - methoxyphenyl)piperazin - 1 - yl]ethyl] - N - (2 - pyridyl)cyclohexanecarboxaminde, FCWAY; F
18 4 - [2’ - (N - 2 - pirydynyl) - p - fluorobenzamido] - ethylpiperazine, MPPF).
Temporal Lobe Epilepsy
Several groups have evaluated 5-HT
1A receptor binding in patients with temporal lobe epilepsy.
61 –
66 All found decreased receptor binding in the mesial temporal lobe containing the seizure focus, in areas of seizure spread, and in the area of the brainstem raphe. Localization was reported to be better than with cerebral blood flow or metabolic rate imaging in some studies.
61,
62 One group found an inverse correlation between abnormal intracerebral activity and binding potential.
63 The most profound decreases in binding potential were in areas of seizure onset; moderate decreases were found in areas of seizure spread, with only mild decreases in areas of interictal activity. These results are promising, but must still be considered preliminary. The importance of correcting for partial volume averaging and for differences in plasma binding related to antiepileptic medications has been emphasized by one group.
65,
66Panic Disorder
One study comparing unmedicated patients with panic disorder to healthy individuals found decreased 5-HT
1A receptor binding in the anterior and posterior cingulate cortices and the raphe area, with no differences in anterior insular, mesiotemporal or anterior temporal cortices.
67 Another reported decreased binding principally in amygdala and orbitofrontal and temporal cortices as well as the raphe area.
68 This study compared binding in an ummedicated patient group with a patient group successfully treated with SSRIs. They reported normalization of binding in all areas except the raphe.
CONCLUSIONS
In conclusion, although the current diagnostic classification defines panic disorder and simple partial seizure disorder as two separate entities, they clearly share similar features and common symptoms and may occur as comorbidities. Rarely, patients diagnosed with panic disorder later show evidence of mesial temporal sclerosis and EEG-proven seizure foci. The close clinical presentations of these two conditions call for a very thorough medical evaluation of those patients with preliminary suspicions of panic disorder and symptoms similar to a seizure aura. If possible, this should include high resolution MRI (with particular attention paid to the mesial temporal cortex) and nuclear imaging. Future studies utilizing new technologies may assist in distinguishing these two entities or in further defining their common pathological base. Nuclear imaging may prove helpful in this endeavor, as very early work may indicate divergent scan findings—especially with receptor-specific ligands.