T here is increasing evidence that combat-related traumatic brain injuries are a frequent occurrence. Recent studies detailing the most common injuries have found that approximately one-half involved the head or neck.
9,
10 The great majority of injuries were due to explosions. Several studies from the Defense and Veterans Brain Injury Center (DVBIC) of soldiers returning from Afghanistan and Iraq document the occurrence of traumatic brain injury (TBI) in many soldiers.
11 –
14 Between January 2003 and February 2005, 59% of returning soldiers treated at Walter Reed Army Medical Center who had been near an explosion while deployed had suffered a traumatic brain injury (44% mild, 56% moderate-severe).
11 Common postconcussive symptoms included headache (47%), irritability/aggression (45%), and difficulty with memory (46%) and attention/concentration (41%).
12 A study of 596 active duty soldiers (all serving full-time at regular duty stations in the United States) found that 96 (16.1%) reported an injury while deployed, for which the symptoms (e.g., alternation in or loss of consciousness) were consistent with TBI.
13 This is similar to an earlier study of active duty soldiers, which found that 13.5% of nonparatroopers reported sustaining a TBI while in the Army.
15 The vast majority of these were mild TBIs, as indicated by either no or only brief loss of consciousness. In most cases, these less severe injuries would not have required medical evacuation.
14 It is well known that civilian mild TBI is underrecognized by both medical personnel and patients, resulting in significant underreporting.
16 There is evidence for a similar situation in the military and concern that combat-related mild TBI may often be unrecognized by both medical personnel and soldiers.
13,
15,
17 Identification of TBI, particularly mild TBI, is often quite challenging. The most common type of injury, and the most likely injury to occur in mild TBI, is traumatic axonal injury (also called diffuse axonal injury).
18 While magnetic resonance imaging (MRI) is more sensitive than computed tomography (CT) in detecting this type of brain injury, even MRI is often negative.
18 –
23 In addition, some areas of injury may become less visible with time.
21 In such cases, MRI in the subacute and chronic stages is less likely to be positive than if acquired immediately following injury. There is increasing evidence that functional imaging (e.g., cerebral blood flow, cerebral metabolic rate) may be considerably more sensitive to the effects of TBI than structural imaging.
23 –
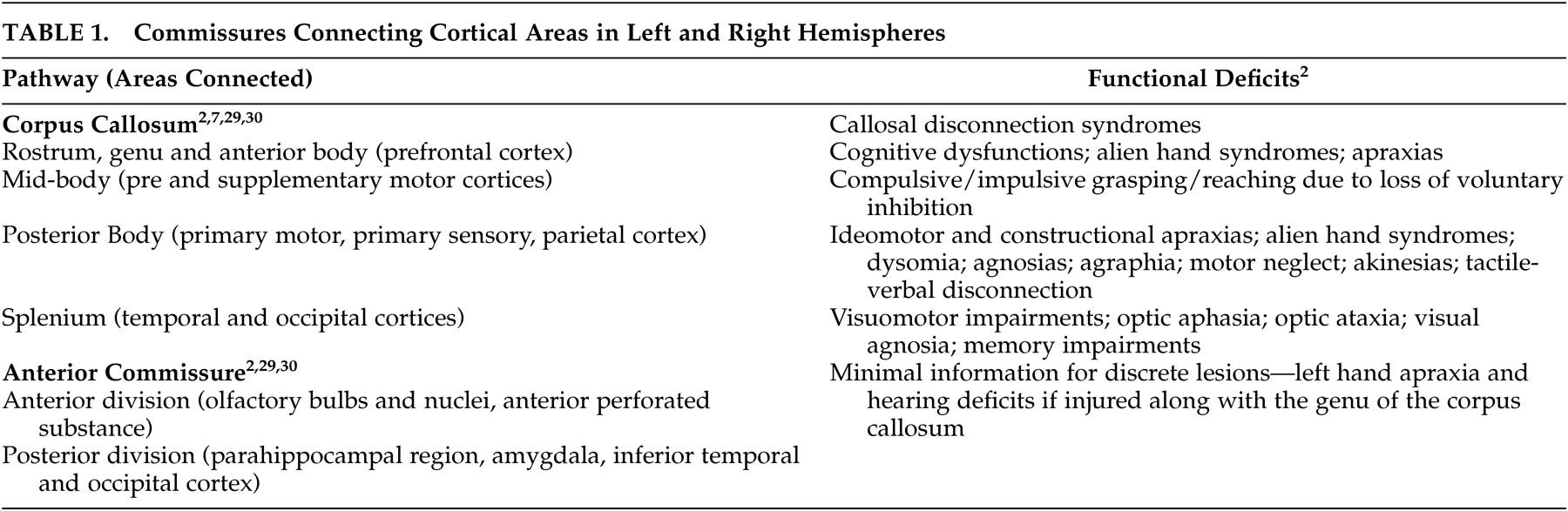
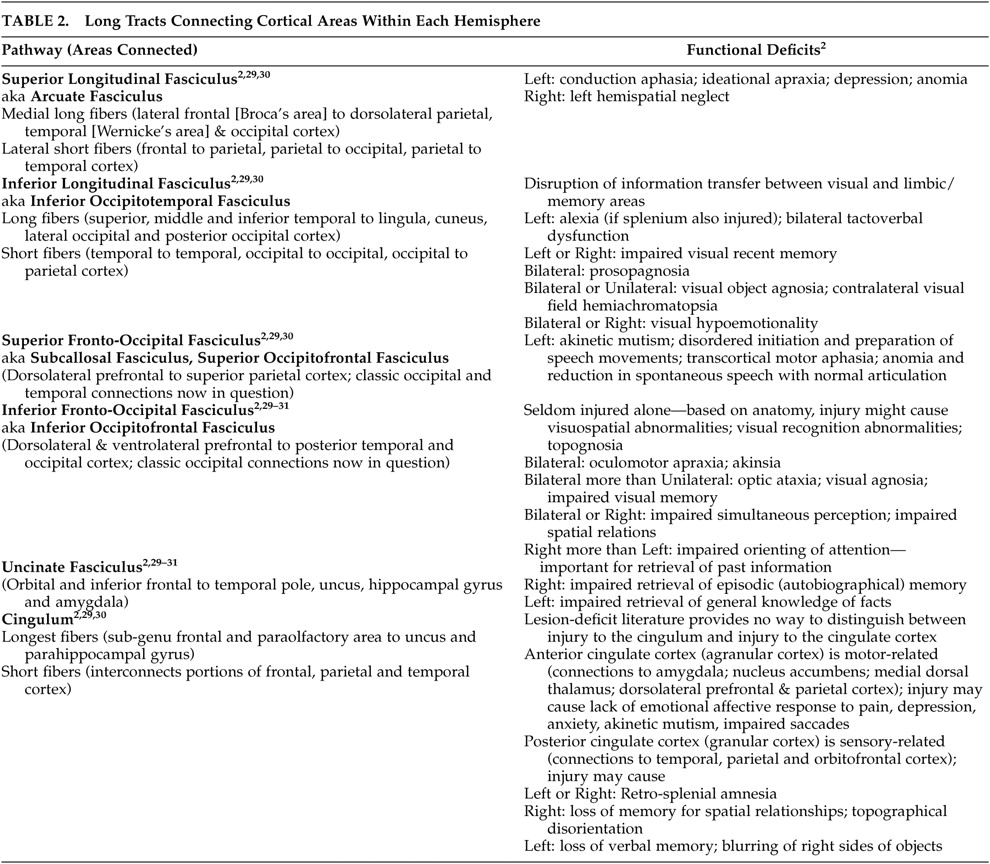
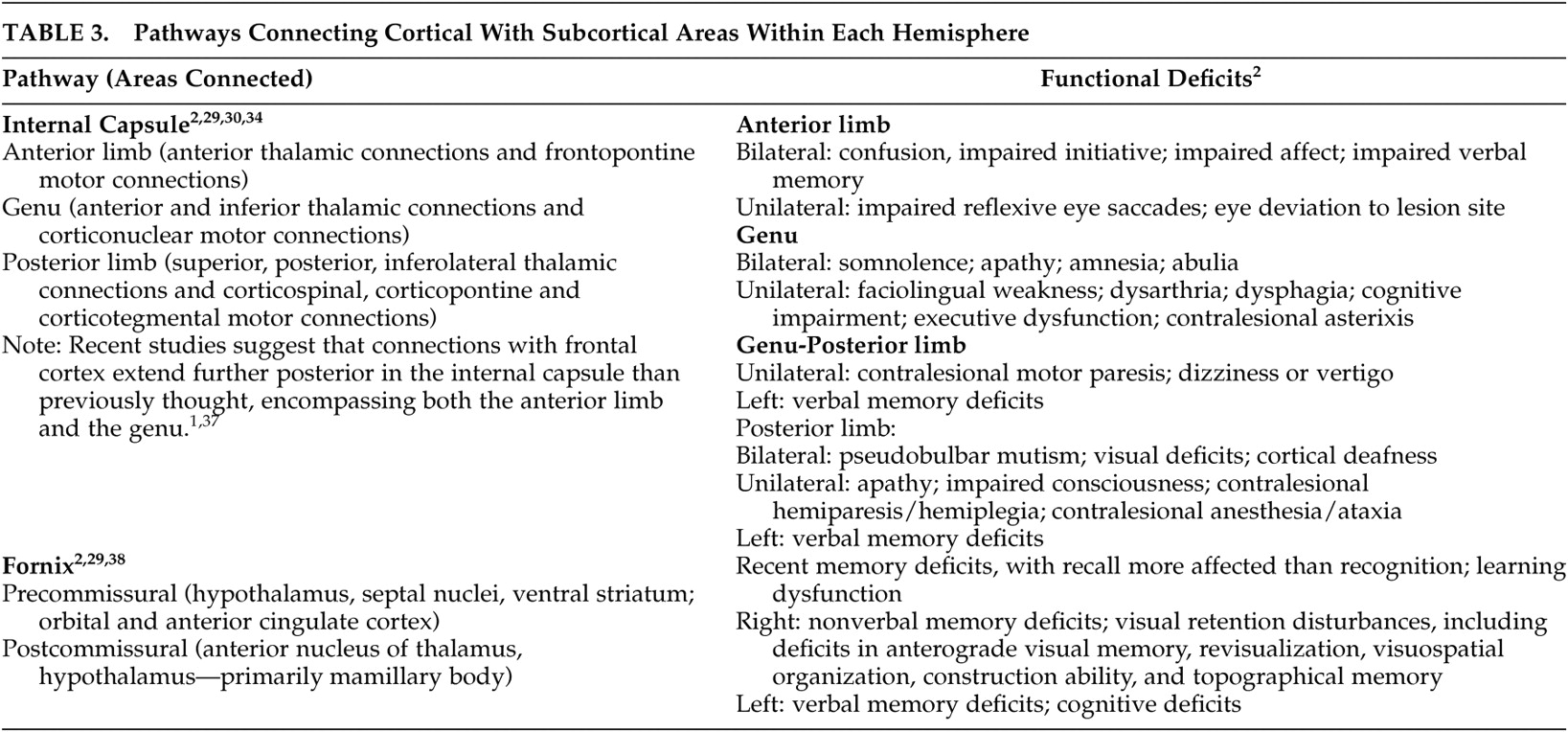
27Even small areas of injury within the white matter may have devastating consequences. Knowledge of the locations of major tracts and the brain areas they interconnect is thus critical for understanding clinical symptoms in the context of TBI. White matter tracts of particular importance in neuropsychiatry include those interconnecting areas of cortex (e.g., corpus callosum, association fiber tracts), those connecting areas of cortex to subcortical structures critical for cognitive/emotional functions (e.g., thalamic radiations) and those interconnecting these subcortical areas (e.g., fornix).
2,
28 Tables 1 to
3 summarize the classic functional anatomy of the major white matter pathways important for cognitive and emotional functioning (
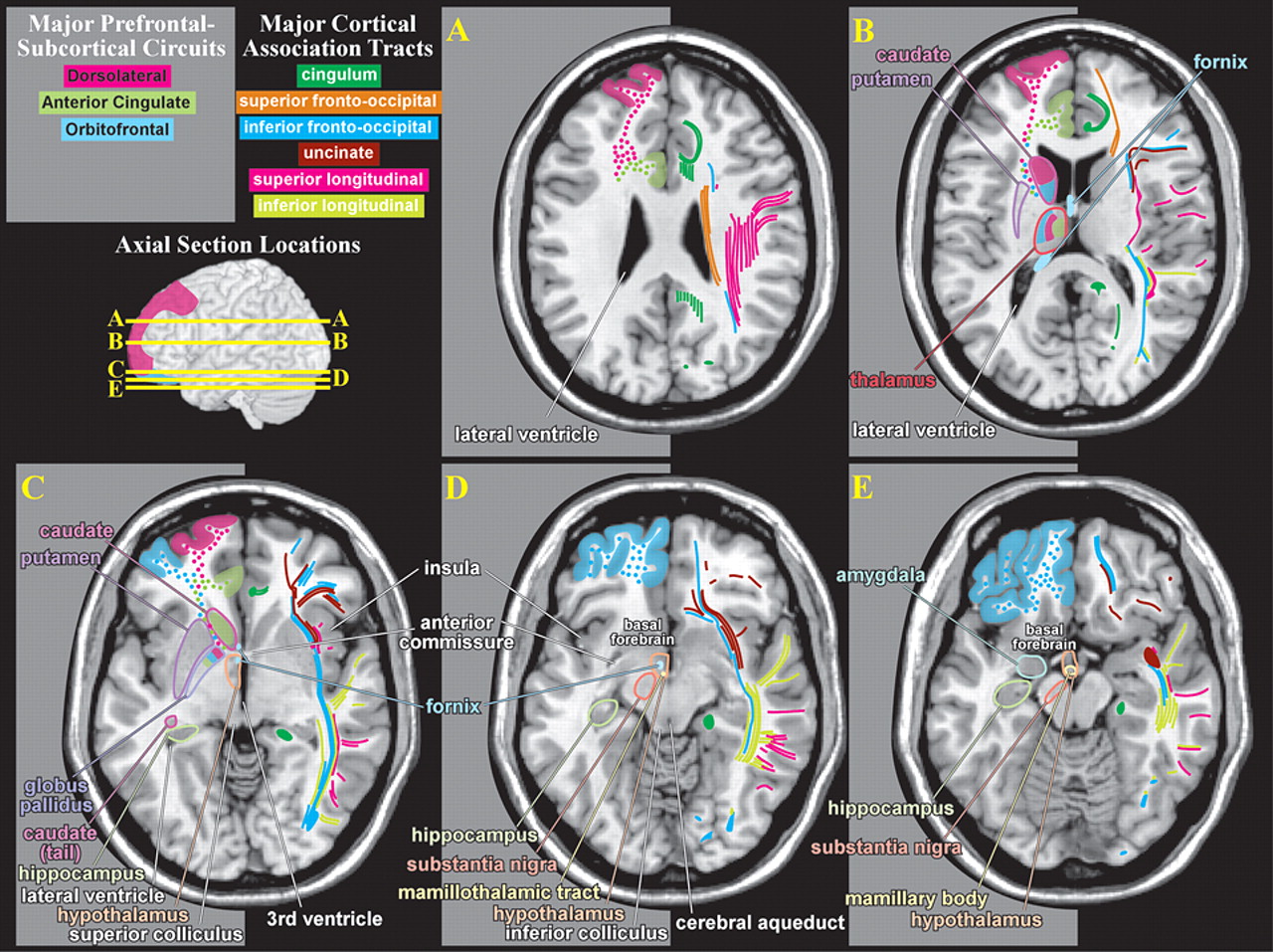
Figure 1 ). They are based on recent studies delineating the anatomy of white matter in humans, primarily using diffusion tensor imaging.
1,
2,
6 –
8,
29 –
33Intriguing results from sophisticated radioisotope tract-tracing studies in nonhuman primates suggests that there may be significant errors in the classic view of cerebral white matter.
34,
35 For example, this work has delineated three different components (in both location and areas connected) of the superior longitudinal fasciculus. A fourth pathway, which this research group considered to be the arcuate fasciculus, was also identified. A recent diffusion tensor imaging study
36 supports the existence of all four of these pathways in humans. Methods for delineating connections within the intact brain are undergoing rapid development and refinement. It is extremely likely that over the next decade, our understanding of the pathways within the brain that are important for cognitive and emotional functioning will change dramatically.
CONCLUSIONS
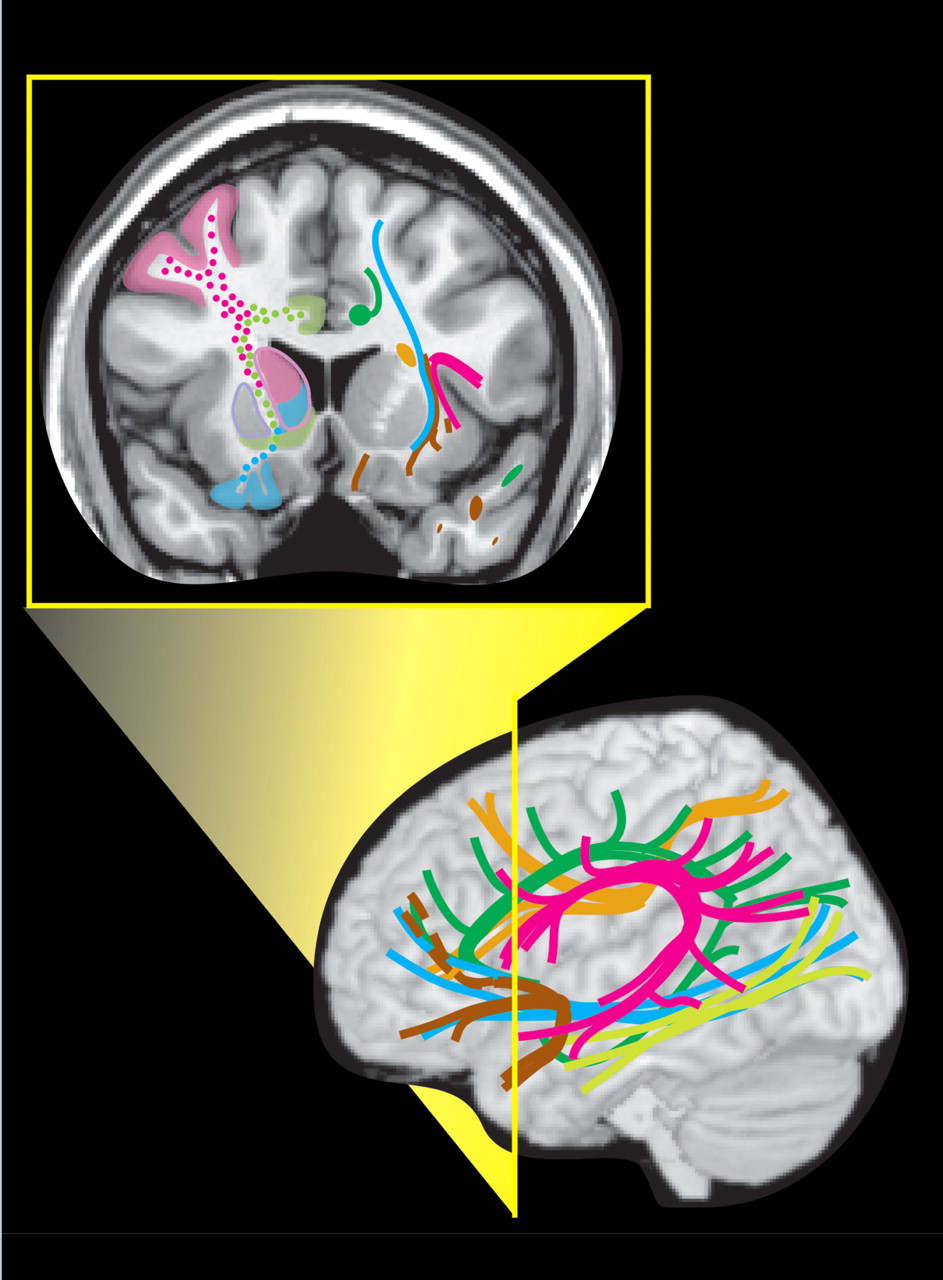
Care must be taken in applying this summary of functional anatomy to individual patients, as studies comparing pathway topography between subjects have shown considerable normal variability ( Figure 2 ).
6 –
8 This is parallel to the normal variation in size, shape, and location of Brodmann’s areas ( Figure 2 ).
3 –
5 This known phenomenon adds a distinct level of uncertainty in predicting individual functional deficits following a brain injury. It should also be kept in mind that in many places multiple pathways travel close together, making it likely that a TBI will affect more than one and produce complex symptom clusters. These symptoms may not become evident for extended periods of time. Atlases and other visual external memory aids can assist clinicians in rapid memory recall of functional circuits and areas for review on patient imaging examinations. Figure 2. Variability (or probability) maps created by transforming the functional anatomy of individual brains into a common anatomic space indicate considerable normal variation.
3–8 On the right is a variability map for primary visual cortex (Brodmann’s area 17), with the number of individuals (out of 10) which overlapped. On the left is a probability map (30% threshold) of the corpus callosum, color-coded by cortical area of fiber origin. Note the large areas of overlap, indicating differences in functional anatomy across individuals.