B uprenorphine hydrochloride is a partial mu opioid receptor agonist and a kappa opioid receptor antagonist.
1 Buprenorphine is commonly used as an analgesic in patients who have severe trauma or are suffering from cancer pain.
2 Though methadone and other pharmacotherapies are available, buprenorphine’s unique pharmacological property makes it well suited for maintenance treatment of opioid dependence.
However, available data suggest increased risk of cerebrovascular events in opioid-dependent patients.
3 –
6 In addition, focal neurological deficits have been reported to occur immediately following heroin injection and up to and beyond 24 hours after heroin abuse. Thus, an agent offering safety in regard to neurological function would be quite valuable for opioid-dependent patients. It has recently been shown that buprenorphine can cause some alterations on the opioid receptors in rat brain regions,
7 which are important indicators of infarct extension in ischemic neuronal damage.
8 Given these in vitro findings indicating the neurotoxic role of buprenorphine, we aim to test the hypothesis of whether buprenorphine is neurotoxic in vivo during cerebral ischemia.
DISCUSSION
Buprenorphine has been in clinical use as an analgesic agent for several decades. It is increasingly used in maintenance therapy of opioid dependence. Opioid-dependent patients are at higher risk of cerebrovascular events and focal neurological deficits.
3 –
6 In vitro studies available to date strongly suggest neurotoxic effects of buprenorphine.
10,
11 However, this in vivo study does not indicate neurotoxic effects of buprenorphine in focal cerebral ischemia model.
A broad spectrum of neuropathological changes has been encountered in the brains of heroin abusers.
3 These changes have been associated either with infections (either due to bacterial spread from bacterial endocarditis, mycoses, or from human immunodeficiency virus infection) or to the other complications resulting from hypoxic/ischemic injuries and related neuronal damage. However, thromboembolism, vasculitis, septic emboli, hypotension, and positional vascular compression are some of the mechanisms hypothesized for respiratory depression and stroke in heroin abusers.
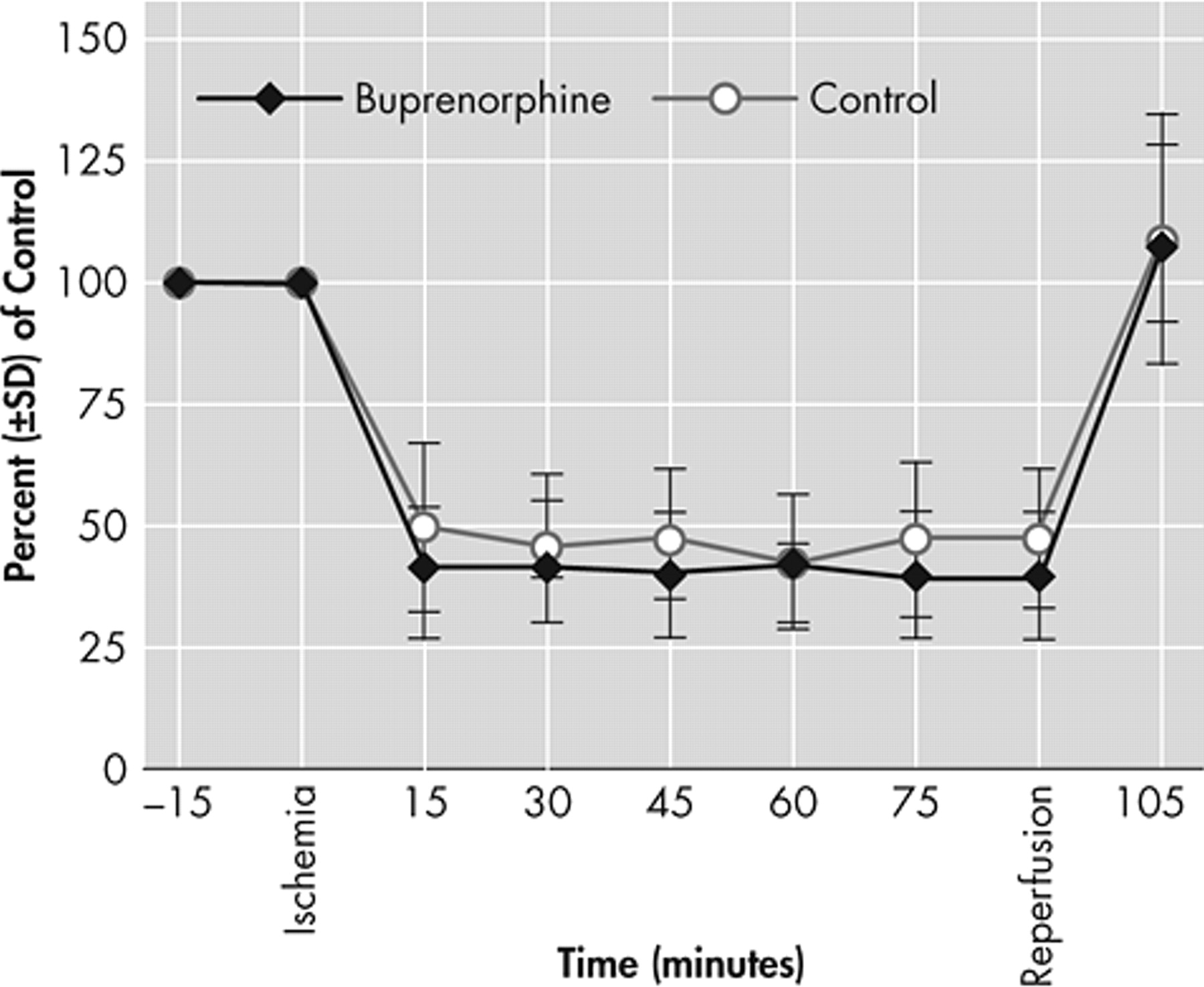
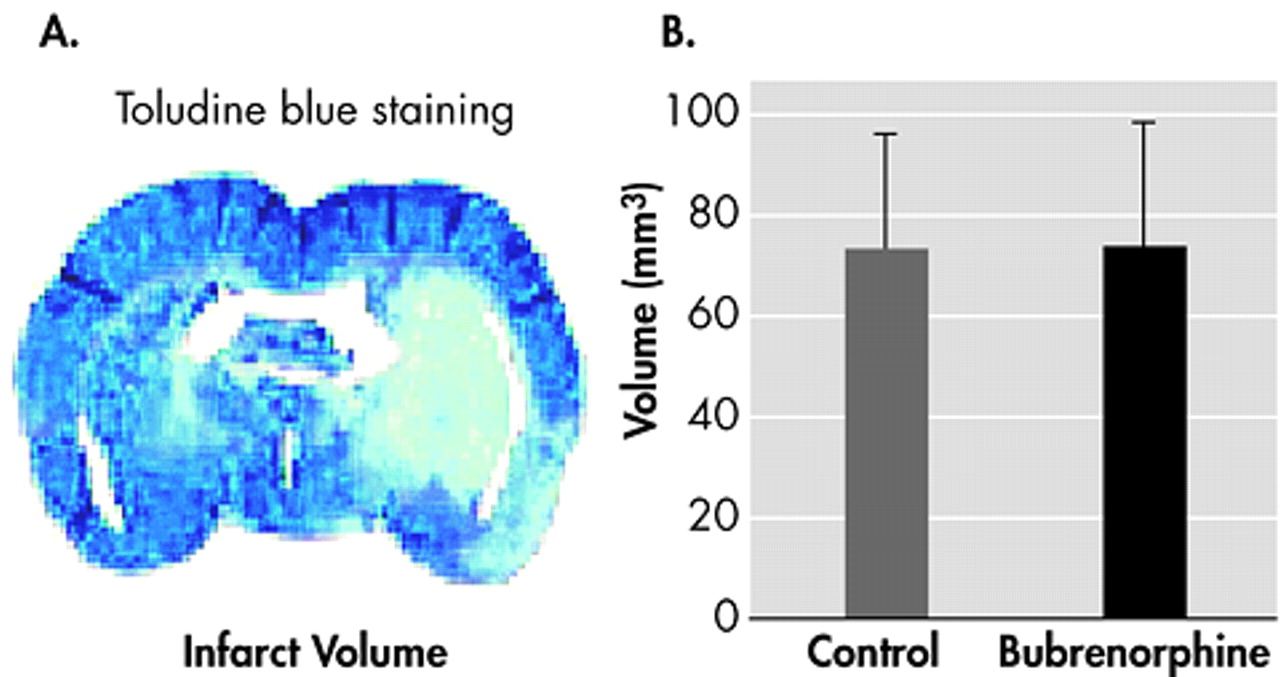
3 Given that the infarct sizes between the buprenorphine and saline-treated groups were not different from each other, the present study demonstrates that single administration of buprenorphine does not cause neurotoxicity in mice with a focal permanent cerebral ischemia model.
It is difficult to explain the reasons behind the difference in findings of this in vivo study and the previous ones in vitro. Yet, while the opioidergic system in the brain has been implicated in the pathophysiology of cerebral ischemia,
8,
12 the studies indicating the neuroprotective role of opioid receptor (KOR) agonists/antagonists are still controversial. Moreover, although recent studies indicated the role of kappa receptor agonists as neuroprotectants in cerebral ischemia,
12 –
14 other studies suggested the role of kappa receptors as mediators for ischemia aggravating effect for endogenous opioids.
15 –
18 Unfortunately, human studies evaluating the neuroprotective role of opioid receptor antagonists failed to find any treatment benefit in clinical studies.
19 –
21Thus, in regard to these controversial results of receptor targeted treatments, it is difficult to explain the difference of this in vivo study from previous in vitro studies only with its kappa receptor antagonist activity which may have balanced its neuroprotection. Moreover, if the same finding can be replicated by the chronic administration of buprenorphine in vivo, adaptative changes leading to receptor up- or down-regulation in opioid receptors may also be discussed as a contributing factor preventing the development of ischemic neuronal damage.
In conclusion, this study provides strong preclinical evidence for the safety of using buprenorphine during the postoperative period following ischemic events, as well as for an alternative maintenance therapy of opioid-dependent patients wherein the risk of cerebrovascular events is increased.