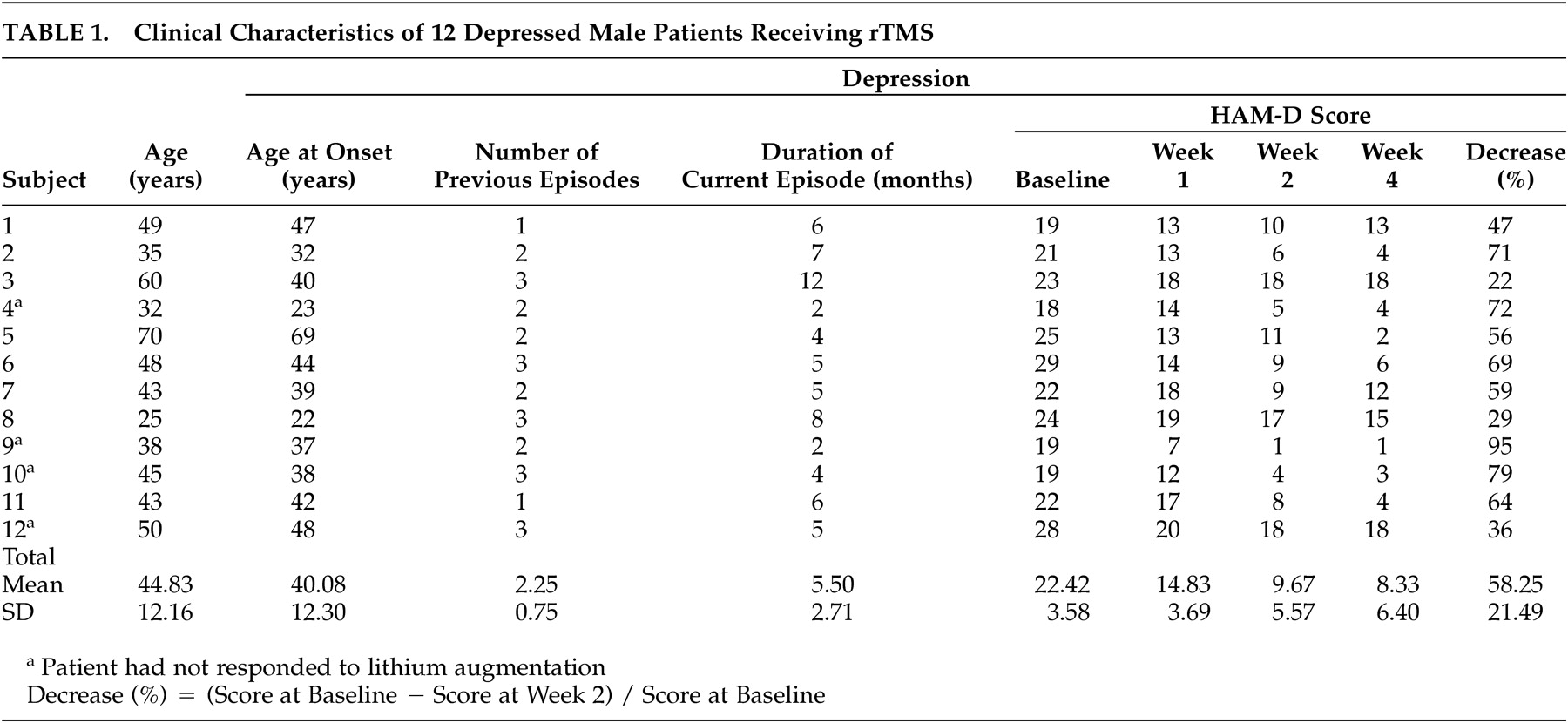
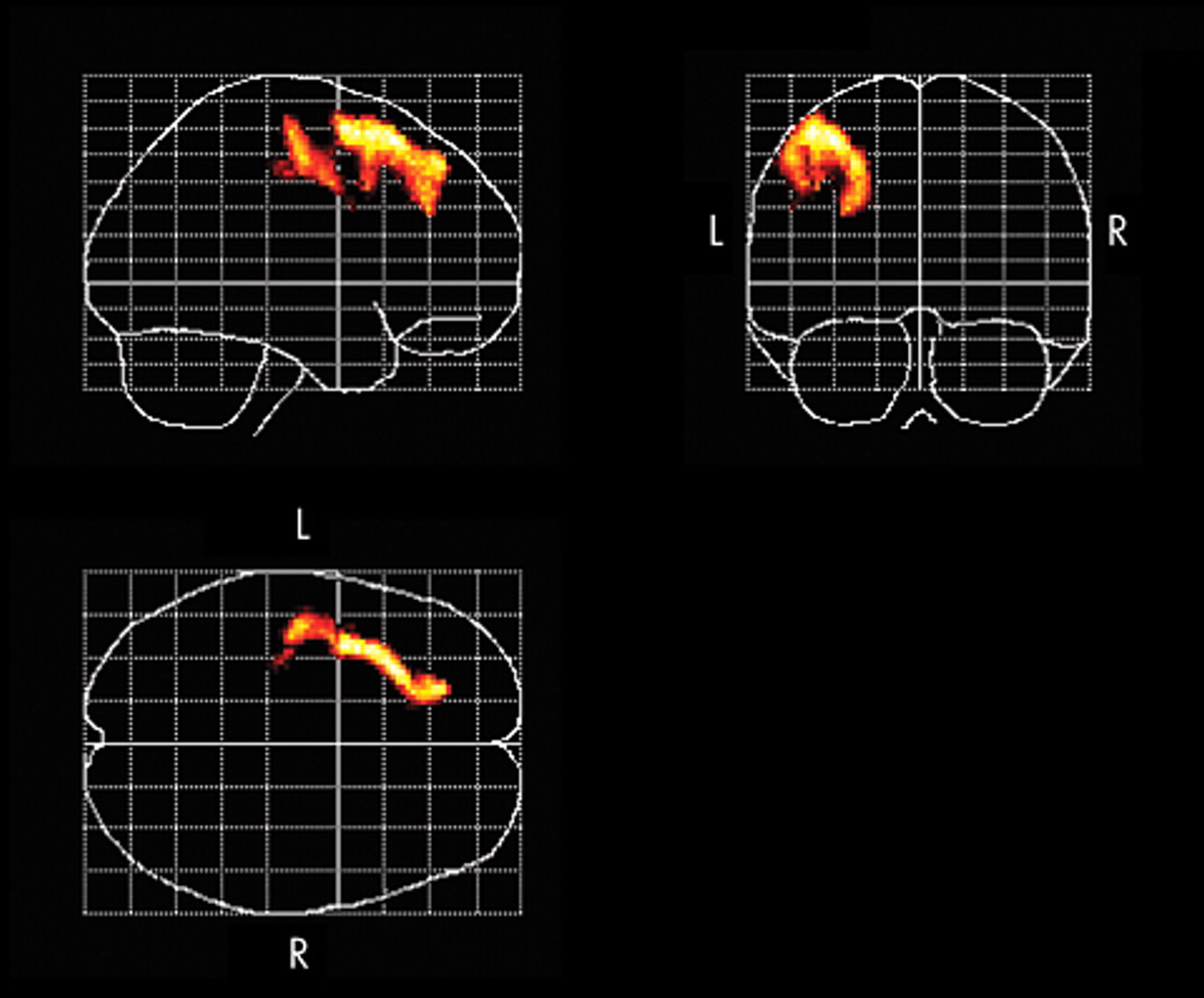
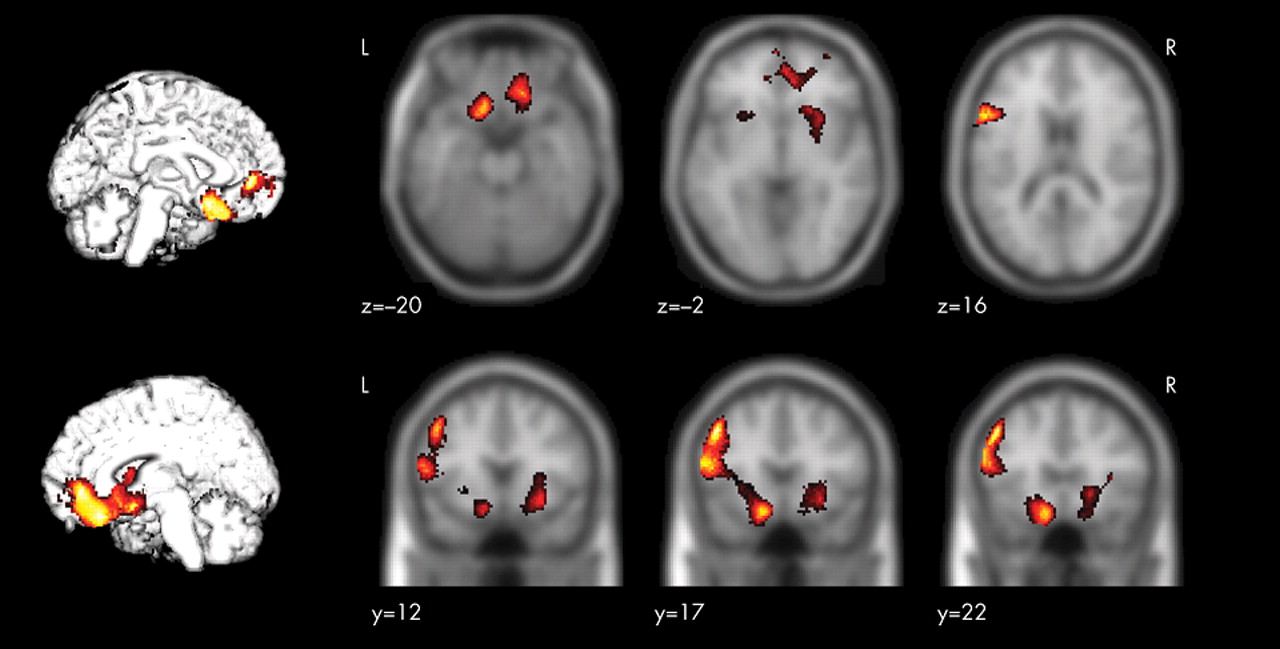
The present study of high-frequency rTMS of the left dorsolateral prefrontal cortex in 12 patients with treatment-resistant depression measured changes in rCBF by SPECT before and after the treatment. After the rTMS treatment, the HAM-D score decreased significantly (mean of −58.25%) and an improvement in the symptoms of depression was observed.
Changes in rCBF
Successful rTMS treatment was associated with a significant increase in rCBF in the left dorsolateral prefrontal cortex in the stimulated region. Furthermore, the present study revealed that there was a relationship between the improvement in symptoms of depression and the increase in rCBF in the left dorsolateral prefrontal cortex, the ventrolateral prefrontal cortex, the orbitofrontal cortex, the anterior cingulate, the left subgenual cingulate, the anterior insula, and the right putamen/pallidum.
It has been suggested that TMS has different effects on neuroanatomical function depending on its frequency, that is, the excitement of the cerebral cortex is enhanced by stimulation from high-frequency TMS and suppressed by stimulation from low-frequency TMS.
10,
30 The activation of the cortex is not limited to the stimulated region but can be transferred to remote regions via the intracerebral networks.
30,
31 According to a study on healthy male subjects after stimulation of the left dorsolateral prefrontal cortex with fast and slow rTMS, increases in CBF in the stimulated regions were observed in both cases. Slow rTMS induced an increase in CBF in the contralateral right caudate body and the anterior cingulate and a decrease in CBF in the ipsilateral orbitofrontal cortex. Fast rTMS applied over the right dorsolateral prefrontal cortex was associated with an increase in the CBF in the stimulated region, the bilateral orbitofrontal cortex, and the left medial thalamus.
32 Speer et al.
30 reported increases in the rCBF in the left-dominant bilateral prefrontal and the limbic and paralimbic regions after a high-frequency rTMS of the left dorsolateral prefrontal cortex in patients with depression. Some studies of high-frequency rTMS for treatment-resistant depression have shown inconsistent results,
23,
24 partly because these studies used different designs in patient selection and treatment methods.
Functional imaging studies show decreases in rCBF and metabolism in the dorsolateral prefrontal cortex
17,
19 and in the left-dominant hypofrontality
18 in patients with depression, and also show changes in rCBF and metabolism according to improvement in symptoms of depression.
20,
22 The results obtained in the present study support those of previous reports, and it is assumed that the direct activation of the cerebral cortex through high-frequency rTMS and the resultant changes in neuroanatomical function associated with the improvement in depressive symptoms are responsible for increasing the rCBF of the left dorsolateral prefrontal cortex after rTMS treatment. Previous studies have demonstrated that the limbic-paralimbic regions are reciprocally connected with the prefrontal and the subcortical regions and are involved in the regulation of mood and affect.
21,
33,
34 In the present study, among patients who responded to rTMS treatment, significant relative increases of rCBF occurred in the ipsilateral dorsolateral prefrontal cortex toward the stimulated region, the limbic-paralimbic regions including the left subgenual cingulate (Brodmann area 25), and the right basal ganglia, whereas no region showed significant relative decrease of rCBF that was correlated with the change of the scores of the HAM-D. Drevets et al.
35 reported a decrease in rCBF and glucose metabolism in the subgenual prefrontal cortex (Brodmann area 25) and a decrease in the volume of the left subgenual prefrontal cortex in patients with familial bipolar and unipolar depression. In addition, there is a report in which marked decreases in the blood flow in the paralimbic regions, especially in the inferior frontal and cingulate cortex, were noted in patients with severe treatment-resistant depression, but there was no decrease in CBF in the parietal or occipital lobe.
36 The anterior cingulate (Brodmann areas 24 and 32) is reciprocally connected with the prefrontal and the limbic-paralimbic regions, and previous reports show that rCBF and metabolism in the anterior cingulate decrease in patients with depression and increase with an improvement in symptoms of depression.
20,
22 In addition, the activation of the rostral anterior cingulate may predict treatment response in depression.
37,
38 The present study showed an increase in rCBF in the anterior cingulate and an improvement in symptoms of depression after successful rTMS treatment of the left dorsolateral prefrontal cortex. Several studies
22,
34,
39 have reported that decreases in rCBF and metabolism in the subgenual cingulate (Brodmann area 25) and the limbic-paralimbic regions such as the anterior insula and the putamen/pallidum are associated with improvement of depression. Acting on the postulation that the subgenual cingulate region is metabolically overactive in treatment-resistant depression, Mayberg et al.
40 reported that deep brain stimulation resulted in a decrease in metabolic activities in this region and in the limbic-paralimbic regions and improved symptoms in these patients. Our findings of increased rCBF in the subgenual cingulate and the limbic-paralimbic regions after successful rTMS treatment are different and have not been previously reported. In most previous studies, rCBF and metabolism were evaluated 6 weeks after the commencement of the trials, whereas in the present study, the rCBF was measured with the SPECT within 48 hours after the completion of the rTMS treatment. Hence, the difference in the timing of the evaluation of the neuroanatomical function during the convalescence from the depression and the manner of the antidepressant treatment may have influenced the findings obtained in the present study.
There are several limitations in the present study. First, a placebo effect due to rTMS treatment cannot be excluded because of the nonblinded design. Second, because pharmacologic treatment was continued during rTMS, the effects of these medications on depression and rCBF should be considered. We believe further studies are necessary to clarify whether the results of the present study are specific findings of the treatment of depression with high-frequency rTMS over the left dorsolateral prefrontal cortex or are due to changes in neuroanatomical function during the time course of convalescence from depression.
In conclusion, we conducted high-frequency rTMS over the left dorsolateral prefrontal cortex in patients with treatment-resistant depression and found changes in rCBF associated with a reduction in symptoms of depression. The results of this study suggest that the manifestation of an antidepressant effect of high-frequency rTMS of the left dorsolateral prefrontal cortex in patients with treatment-resistant depression is related to changes in the neuroanatomical function of the left dorsolateral prefrontal cortex as well as of the ipsilateral subgenual cingulate (Brodmann area 25), the ventrolateral prefrontal cortex, the orbitofrontal cortex, the anterior cingulate, the anterior insula, and the right putamen/pallidum.