L ipoid proteinosis, also known as
Hyalinosis cutis et mucosae or Urbach-Wiethe Disease, is a rare hereditary disorder transmitted by an autosomal recessive gene.
1 –
2 From the time Urbach and Wiethe
3 gave the first clear description of the disease, a cumulative total of 250
1 to 300 cases
4 –
6 have been reported in the literature. The disorder is a systemic illness characterized by depositions of storage material in the mucous membranes.
4,
7 –
11 The most classic symptoms are hoarseness of speech, often from birth or infancy,
7,
12 and skin lesions.
12 –
14 There is considerable clinical variability among lipoid proteinosis subjects
2 and lesions may increase in severity and extent with age.
15 The most consistent clinical features for a diagnosis have been a hoarse voice and a thickened sublingual frenulum leading to restricted tongue movement.
2 Bilateral, circumscribed, and symmetrical calcification in the medial temporal regions are common
4,
9,
16 –
17 and are associated with neuropsychiatric sequelea including seizure disorder
9,
16,
18 and psychotic symptoms.
6,
19 –
20 Medial temporal regions encompass the amygdala and studies on patients with lipoid proteinosis contributed to our understanding of the role of the amygdala in fear processing and social cognition.
17,
21 –
26 The lipoid proteinosis subjects of Adolphs et al.
21 and Calder and Young
27 showed significant impairment in recognizing fearful expressions, although individual performances ranged from severely impaired to essentially normal. In addition, while most subjects were impaired in recognizing other negative expressions in addition to fear, they were not impaired in recognizing happy expressions. Lipoid proteinosis has been associated with difficulty in coping with emotionally loaded memory
28,
29 and impaired executive control over social behavior.
17There has, however, been significant variability in neuropsychiatric and neuropsychological findings across studies. In some studies there has been no evidence of psychiatric symptomatology.
30 –
32 Siebert et al.
28 studied 10 people with lipoid proteinosis, six with bilateral calcification of the amygdaloid complex, and did not find that lipoid proteinosis necessarily impaired the recognition of basic emotions such as fear and anger. Lipoid proteinosis has been associated with both intact intelligence
9,
17,
20,
30,
33 and mental retardation.
14,
16,
34 –
36While there is true heterogeneity within the lipoid proteinosis population,
2 which may represent a variable and slowly progressive degenerative process in the brain,
28 some of the variation across the studies may be explicable on the basis of relatively small sample sizes and other methodological problems (e.g., different methods of assessing symptomatology). The current study is, to our knowledge, the largest systematic study of psychiatric diagnosis and neuropsychological measures in lipoid proteinosis. In view of evidence of amygdala pathology and prior findings of associated neuropsychiatric impairment, we hypothesized that this population would be characterized by increased rates of mood and anxiety disorders and impairments in processing emotional expressions.
METHOD
Thirty-four adult South African subjects with lipoid proteinosis (ages 17–63 years) participated in this study. Two were patients attending a large state hospital in Cape Town, five were from the greater Johannesburg, South Africa/Pretoria area, and 27 lived in the impoverished and rural Northern Cape. Forty-seven controls from the Northern Cape (matched for home language, socioeconomic status, age, and matched as closely as possible for years of education and gender) completed the same batteries. Ethics approval was obtained from the University of Stellenbosch, Tygerberg Hospital, and abided by the Helsinki, Finland, declaration guidelines of “good clinical practice.” All subjects gave informed consent. Six participants (two with lipoid proteinosis and four matched controls) were 17 years old and they and their guardians gave consent. Three children with lipoid proteinosis under 16 years were excluded from this study.
The diagnosis of lipoid proteinosis was established through genetic and clinical means. Subjects were examined by use of standardized neuropsychiatric and neuropsychological measures. The battery was designed to assess neuropsychiatric symptoms and neuropsychological dysfunction previously reported in the literature in association with lipoid proteinosis (e.g., psychosis, depression, problems with emotional recognition, and memory and executive functioning). Tests were not administered if the subjects were unable to complete them validly (e.g., due to psychosis or illiteracy). Subjects were assessed in their home language on the following neuropsychiatric measures: Mini International Neuropsychiatric Interview (MINI+),
37 Positive and Negative Syndrome Scale (PANSS),
38 and Montgomery-Asberg Depression Rating Scale (MADRS).
39 They were assessed on neuropsychological measures including the Wechsler Abbreviated Scale of Intelligence (WASI) except for the vocabulary subtest.
40 This was substituted with the vocabulary subtest from the WAIS-III scaled score, using the Human Sciences Research Council of South Africa’s Afrikaans translation and adaptations for South Africa.
41 Two people with lipoid proteinosis had already completed the South African Wechsler Intelligence Scale-Revised (SAWAIS-R) and did not do the WASI.
42Subjects were also assessed with the Benton Facial Recognition (short form)
43 and the Ekman Facial Emotional Recognition.
44 This test consists of 110 photographs of people displaying either a neutral expression or one of six emotions (happiness, sadness, fear, anger, surprise, or disgust). Subjects must try and match one of the seven options to the expression they see. In addition, subjects were assessed with the Controlled Oral Word Association Test (COWAT); the Rey Auditory Verbal Learning Task (RAVLT); subtests from the Wechsler Memory Scale—Revised,
45 including Logical Memory, translated into Afrikaans with the names changed to local geographical settings (e.g., Okiep instead of Boston); Complex Figure of Rey (Copy and Immediate Incidental Recall); draw a clock; Hooper Visual Organization Task; Trail Making Tests A and B; and the Ruff Figural Fluency Test.
Statistical Analysis
As exemplified in the
Table 1 vignettes, the Northern Cape subjects differed significantly from the non-Northern Cape lipoid proteinosis patients on a number of demographic parameters (i.e., years of education, level of employment). Thus non- Northern Cape lipoid proteinosis subjects were excluded from further neuropsychiatric and neuropsychological analyses. Data from Northern Cape lipoid proteinosis subjects and controls were compared with analysis of variance (ANOVA) when normally distributed and with Mann-Whitney analyses when not normally distributed. Significance was set at p<0.05.
RESULTS
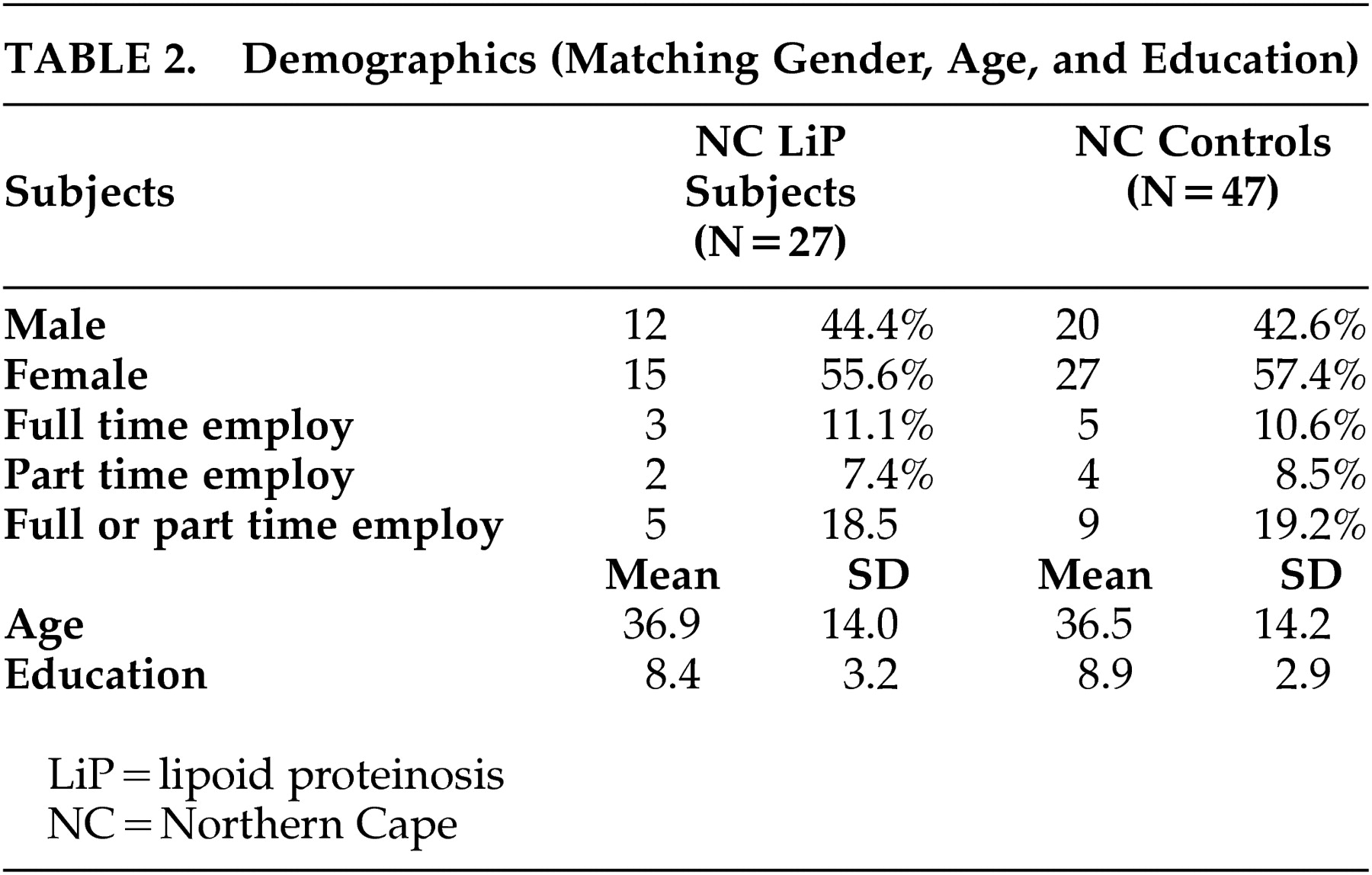
In the Northern Cape lipoid proteinosis group (N=27), there were 12 males (44.4%) and 15 females (55.6%) (
Table 2 ). The mean age was 36.89 years (SD=14.0), the mean education was 8.37 years (SD=3.2), and less than 12% were in full-time employment. The Northern Cape control group had 20 males (42.6%) and 27 females (57.4%), with a mean age of 36.51 years (SD=14.2) and a mean education of 8.94 years (SD=2.93), and 10.6% were in full-time employment. Twenty-seven percent (27%) of the Northern Cape lipoid proteinosis group reported seizures.
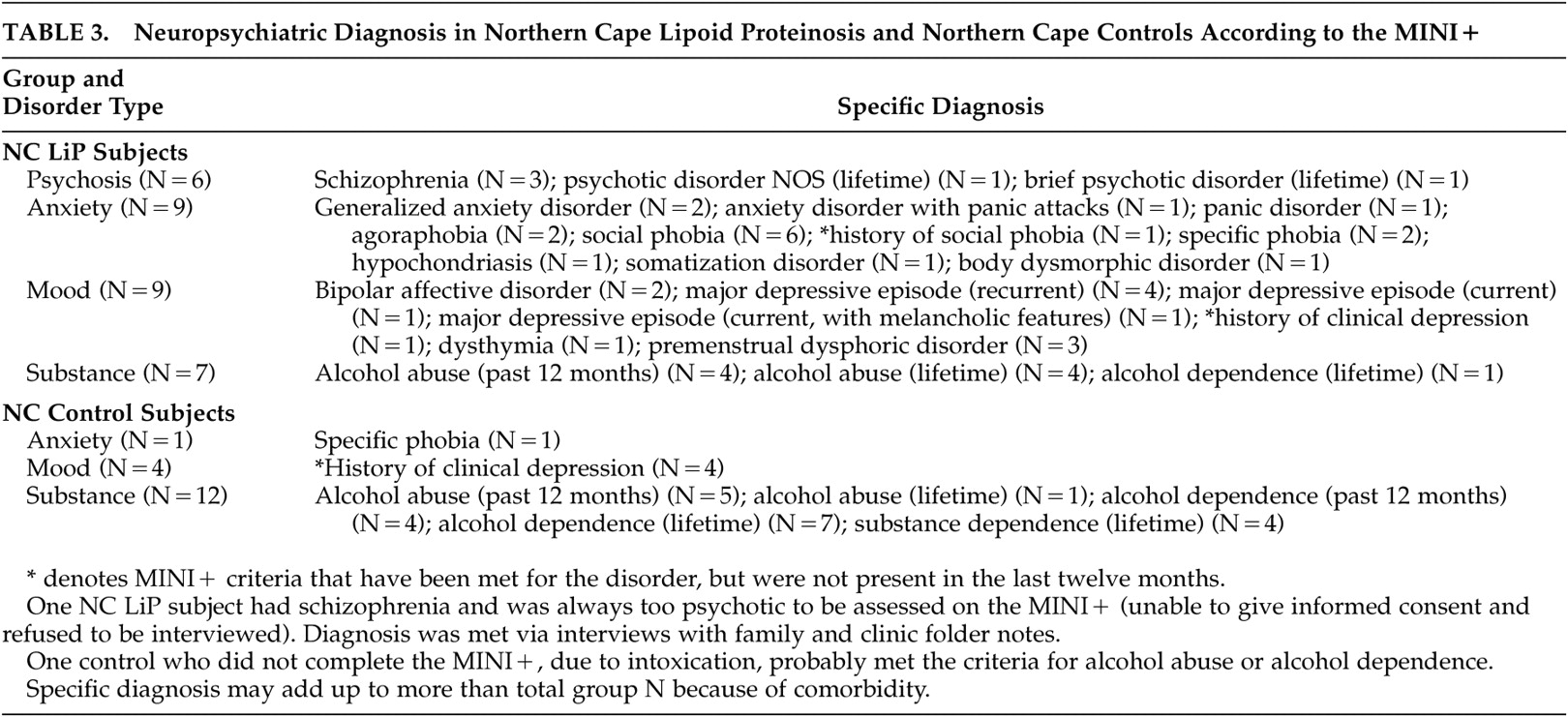
Neuropsychiatric diagnoses were compared in lipoid proteinosis subjects from the Northern Cape (N=27) and controls (N=47). In the Northern Cape lipoid proteinosis group, anxiety disorders (33%), mood disorders (33%), psychosis (22%), and schizophrenia (15%) were common (
Table 3 ), and in all cases significantly more prevalent than in the control group (p<0.01). Similarly, the Northern Cape lipoid proteinosis group was significantly more likely to have higher scores on the MADRS and on the negative symptoms scale of the PANSS. There was no significant difference between the groups for alcohol abuse (26% in both); 58% of lipoid proteinosis and 60% of control adult men abused alcohol (MINI+). Adults with lipoid proteinosis were significantly less likely to live with partners or to have children.
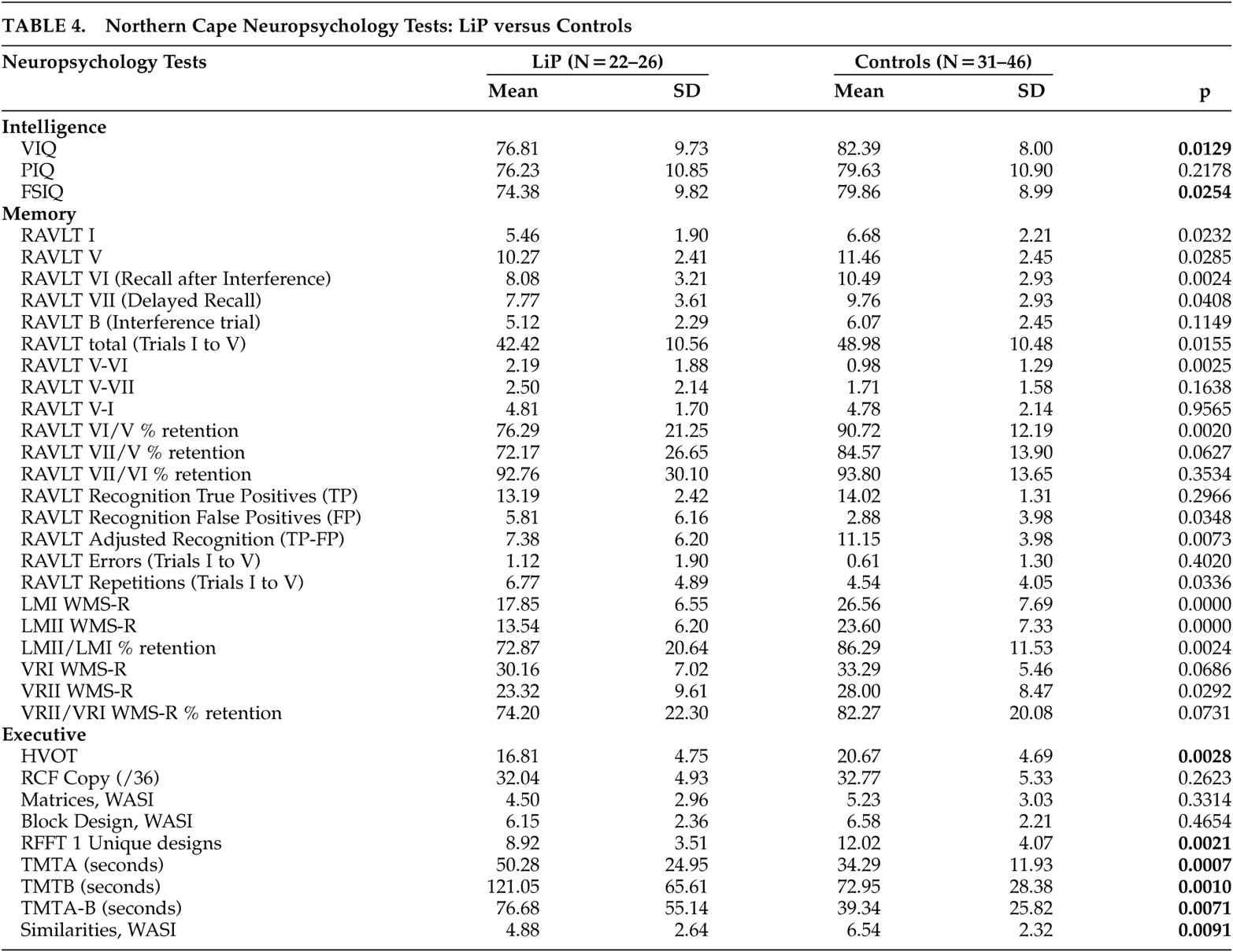
The mean IQ for both the Northern Cape lipoid proteinosis subjects and the Northern Cape controls (including many educationally deprived participants) was markedly poorer than established norms (
Table 4 ). When comparing the lipoid proteinosis to the control subjects the following was evident: of the three WASI tests and Vocabulary test from the WAIS-III, only Similarities was significantly different between the two groups (controls significantly better than lipoid proteinosis subjects).
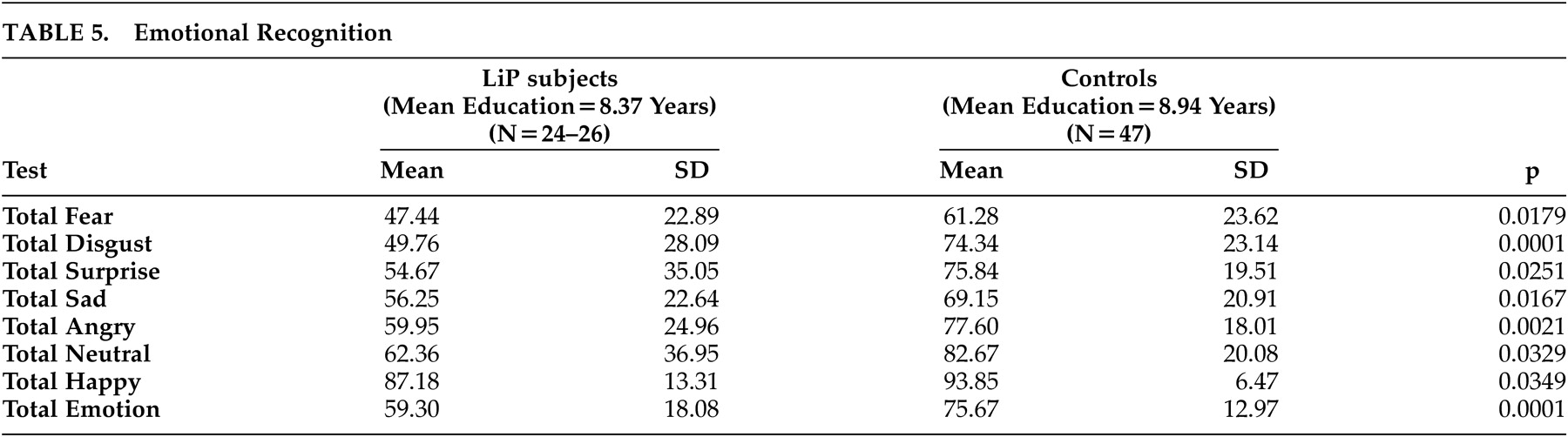
Northern Cape lipoid proteinosis subjects were significantly less likely to recognize correctly the emotional expressions—fear, anger, disgust, surprise, sadness, happiness and neutrality—than the matched Northern Cape controls (
Table 5 ). On basic face recognition, there were no significant differences on the Benton Facial Recognition Test, unless presented with less lighting or from a different angle. In terms of neuropsychological testing, there were no significant differences across Northern Cape lipoid proteinosis subjects and controls for the two tests of language (COWAT and Vocabulary, WAIS-III). However, the Northern Cape lipoid proteinosis group scored significantly worse than controls on several tests of memory, including digits forward and backward, auditory new learning on RAVLT, retention after distraction, the recognition task, the number of repetitions in new learning, both immediate and delayed paragraph recall, as well as the percentage of the story that was retained, and delayed recall of the visual reproduction designs. There were no significant differences in either the forewarned or incidental immediate visual recall tasks (
Table 4 ).
On selected executive tasks, there were no significant differences on tests of planning (draw a clock, copy of complex figure, WASI Matrices, or block design) between the Northern Cape lipoid proteinosis group and controls, although the Northern Cape lipoid proteinosis group did significantly worse on visual organization (
Table 4 ). However, for tasks of design fluency, switching and dual sequencing, and abstract thinking, the control group performed significantly better. Thus, controls performed significantly better than Northern Cape lipoid proteinosis subjects on memory and specific executive abilities (design initiation, switching, visual organization, and abstract thinking), but language tasks and other executive skills (planning) appeared unaffected by lipoid proteinosis.
DISCUSSION
These findings represent the largest report to date of neuropsychiatric and neuropsychological data in people with lipoid proteinosis. Compared with controls, a sample of people with lipoid proteinosis in the Northern Cape had increased rates of mood, anxiety, and psychotic disorders as well as greater symptom severity, a decreased ability to identify both positive and negative emotional expressions, and significant impairment on memory and executive (specifically design fluency, switching, and abstract thinking) functions. The findings here confirm many of the observations made in previous reports on neuropsychiatric symptoms and neuropsychological impairment in smaller groups of lipoid proteinosis subjects.
21 –
27A larger sample provides us with some additional information; for example, we were able to demonstrate that there is impairment not only in processing of negative emotions, but also in processing of positive emotions in lipoid proteinosis. It can be hypothesized that such deficits have a significant impact on functioning in lipoid proteinosis. Nevertheless, we noted that the people with lipoid proteinosis were able to interact appropriately with us, possibly suggesting the presence of compensatory mechanisms.
28 However, the negative impact of impaired cognitive and affective processing may help explain why lipoid proteinosis patients were less likely to be living with partners and had fewer children than controls.
There are only occasional inconsistencies between our data and those reported previously. One report did not find memory or executive difficulties in lipoid proteinosis.
28 This may well reflect restriction of that sample to subjects with IQ predominantly in the average to above average ranges. Indeed, the overall picture of lipoid proteinosis described here is also consistent with previous findings of marked inter-individual variability within the lipoid proteinosis population, perhaps reflecting differences in extent of intracranial calcification in this condition
7 or in the extent of comorbid seizures (although we did not find significant differences in those with and without seizures).
It is also worth considering psychosocial contributors to variance in the data here. We noted significant difference in intelligence and education between lipoid proteinosis subjects living in the relatively impoverished Northern Cape and a smaller group who lived elsewhere. Indeed, notable findings here included the high prevalence of alcohol abuse and low neuropsychological performance of normal controls living in the Northern Cape. Much remains to be done in this setting in order to reverse current psychosocial circumstances and their negative impact on mental health.
The amygdala plays a key role in mediating emotional responses,
17,
19,
21,
27 particularly those related to possible danger and threat.
23 –
26 The amygdala is also important in the modulation of attention, perception, learning, and memory,
46,
47 as well as in a number of different psychopathologies.
48 –
53 The data here on neuropsychological dysfunction, as well as increased psychotic, mood, and anxiety disorders, is consistent with temporal involvement and with the known high prevalence of temporal calcifications in lipoid proteinosis.
9,
16,
19,
28,
31 Patients in the current study live in remote rural areas and brain imaging was not available; such work could in the future shed more light on the findings here.
Acknowledgments
This research was supported by the Medical Research Council of South Africa.