T he first 20th-century paper on tick-borne infections was written in 1922.
4 Since that time, evidence regarding assessment and treatment of these infections has gradually accrued. The most common of tick-borne infections is Lyme disease (Lyme borreliosis, LB).
10 In the United States, the spirochete,
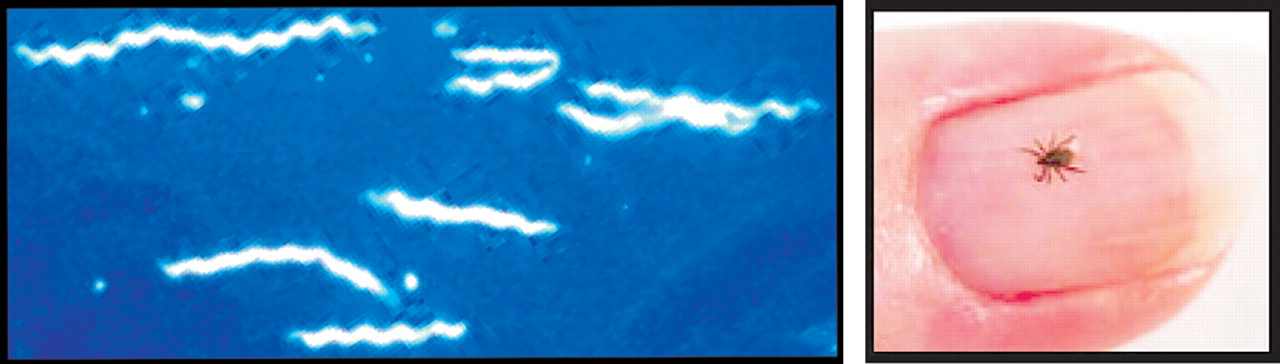
Borrelia burgdorferi sensu stricto, is the causative agent (
Figure 1 A ). High risk of exposure is principally in the eastern United States, with limited pockets of high risk on the western coast. Additional
Borrelia species are prominent in Europe and northern Asia, with many more confirmed cases. According to the Centers for Disease Control web site, annual cases reported in the U.S. have more than doubled between 1991 (<10,000 cases reported) and 2005 (23,305 cases reported). The
B. burgdorferi spirochete is transmitted through the bite of the blacklegged (
Ixodes ) tick (
Figure 1 B ). One report noted that the tick must remain in place for 24–48 hours to transmit enough spirochetes to produce infection in the immunologically healthy individual.
5 Field mice are the environmental host of choice, although birds, squirrels, and other small mammals can also act as hosts. Laboratory testing is via the ELISA titer followed by Western blot test for confirmation. Care must be taken in eliminating negative test results early as the rash may appear before serologic conversion occurs and antibodies may remain long after acute infection has resolved (i.e., months or years). Repeat testing may be needed.
5 Culture of the organism is very challenging as it is very slow growing, appears in small numbers, is tissue based, and needs special culture media. Even polymerase chain reaction-based techniques do not often demonstrate positive results. Thus, most diagnostic investigations rely on antibody testing for a definitive answer.
4,
6 Early Localized and Early Disseminated Stages
The most common early manifestation (3–30 days after exposure) is a skin lesion at the site of the tick bite. This may be a diffusely reddened slowly expanding rash, or less commonly, the lesion may have a “bull’s eye” appearance (60–80% of cases). This rash is termed erythema migrans. This may be accompanied by fever, lymphadenopathy, arthralgia, and/or myalgia.
5,
7,
8,
9 Later manifestations are due to spread of the spirochetes either under the skin or by way of the bloodstream to organs such as the brain, heart, and joints.
10 In the U.S., the causative agent is
Borrelia burgdorferi sensu stricto. According to a recent review article, in these U.S. cases erythema migrans lesions are commonly present, less than 10% of Lyme disease cases develop Lyme neuroborreliosis, painful radiculitis is rare, Lyme arthritis is common, and meningitis is present in the majority.
4 In Europe, where most cases are due to
Borrelia garinii or
afzeli, erythema migrans lesions are uncommon, more than 35% of Lyme cases develop Lyme neuroborreliosis, painful radiculitis is common, Lyme arthritis is rare, and meningitis is present only in the minority of cases.
4Lyme neuroborreliosis in the U.S. is characterized by a subacute meningitis (facial nerve palsy may be present) sometimes accompanied by headache, myalgia, malaise, numbness, tingling, and/or mild cognitive impairment. Lyme neuroborreliosis in Europe usually presents with a painful radiculitis (Bannwarth’s syndrome, Garin-Bujadoux syndrome). Peripheral neuropathy may be present with either form of Lyme neuroborreliosis.
4 Certainly, the cranial neuropathies are the most easily identified of the classic symptoms. Although seventh nerve palsy is the most frequent, other cranial nerve involvements have been reported, included cranial nerves 4, 5, 6, and 8. Understanding of this predilection to the cranial nerves has been slow in development, as the only known animal model to develop nervous system involvement (rhesus monkeys) developed peripheral nervous symptoms only.
6 In Europe, encephalomyelitis has been documented more frequently in both the acute and later stages of Lyme neuroborreliosis illness. MRI hyperintensities in cortical, subcortical, and/or cerebellar hemispheres are commonly reported. The CNS is more severely affected by the strains common in Europe.
6,
11,
12,
13 For example, in one case report the patient had involvement of the spinal nerves and roots giving a polio-like presentation with flaccid paraparesis and MRI enhancement of the cord.
14Multiple case reports have documented the diversity of clinical presentations that are possible in Lyme neuroborreliosis, as well as the challenges in differential diagnosis that result. CNS Lyme disease can result in a pleocytosis, high cell counts, and atypical lymphoid cell in the CSF. This can look remarkably similar to primary CNS lymphoma non-Hodgkin’s type on lumbar puncture.
9,
15 Although less common, parenchymal involvement can and does occur, particularly if the initial infection is left untreated.
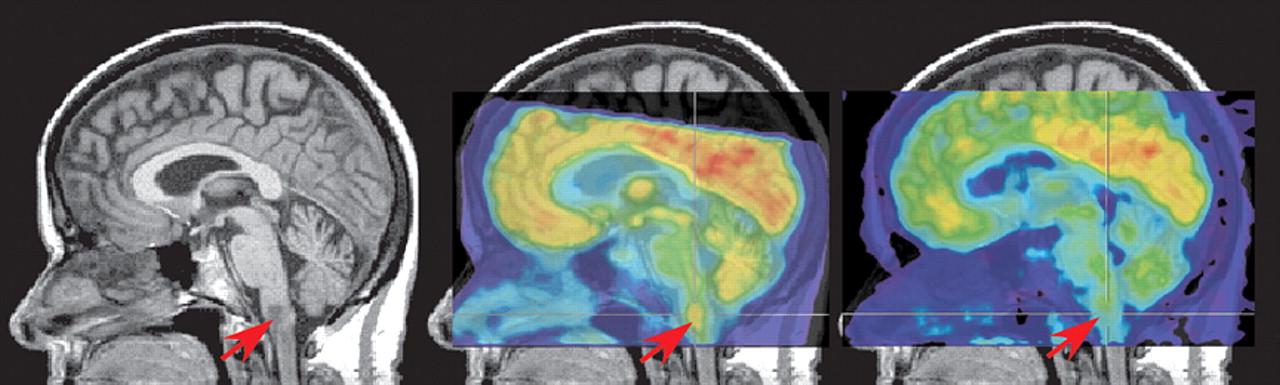
1,
16 In both of these cases, a brainstem lesion was found that was hyperintense on T2 MRI, hypointense on T1 MRI, and hypermetabolic on [
18 F] fluorodeoxyglucose positron emission tomography (FDG-PET), findings that are consistent with neoplasm (
Figure 2 ). Both cases were successfully treated with antibiotics and follow-up neuroimaging was performed. In one case, partial resolution of the lesion was found on MRI (PET was not performed).
16 In the other case, the lesion was still present on MRI, but was no longer hypermetabolic on PET (
Figure 2 ).
1 The authors of both studies noted that hypermetabolism can be associated with both neoplasm and inflammation. Other cases of brainstem
Borrelia infection closely mimicking malignancy have included a child with a mass extending from the pons to C4.
17 Most interestingly, as the authors of this case reflect, this presentation was not only atypical in the mass evident on MRI, but also in the atypical clinical presentation (i.e., nausea, vomiting, weight loss, headache, and nuchal restriction). Ninety percent of childhood cases present with acute meningitis, facial paralysis, headache, and disturbed cognitive function.
Structural neuroimaging in Lyme disease is not diagnostic, as many patients will have normal examinations.
18,
19,
20,
21,
22 Contrast enhancement of cranial nerves (particularly CN VII) has been reported. Small areas of abnormality in the white matter that are hyperintense on T2W MRI, similar to those seen in multiple sclerosis, may be present. A recent prospective study of patients with neuroborreliosis reported multiple sclerosis-like lesions in 80% (12/15) of neuroborreliosis patients with focal symptoms compared to 0% (0/5) of neuroborreliosis patients with nonfocal symptoms.
22 In stark contrast to what is found in multiple sclerosis, however, several measures of tissue integrity were normal in both areas of brain containing lesions and areas (both gray matter and white matter) that did not. Thus, the magnetization transfer ratio, mean diffusivity, and fractional anisotropy of these regions were not different between the patients with neuroborreliosis and the healthy comparison group. As the authors of the study noted, these findings suggest that neuroborreliosis is associated with little structural damage.
As is true in other conditions, functional imaging may show abnormalities not apparent on structural imaging. A recent retrospective study of patients with neurological symptoms attributed to Lyme disease found that 83% (19/23) had areas of mild-moderate hypometabolism on FDG-PET scans obtained either just prior to or just after commencement of antibiotic therapy.
20 The most common location was the temporal lobe (17/23) in one (5/17) or both (12/17) hemispheres. Hypometabolism in frontal and/or parietal cortex and/or subcortical areas was also present in some cases. The authors noted that they found no correlation between any specific metabolic pattern and age, duration of illness, prior treatment, or current medications. Follow-up PET scans obtained in three patients were congruent with clinical state: one patient had no significant changes in either symptoms or PET scan; two patients had a worsening of symptoms and greater areas of abnormality in their PET scans. Previous SPECT scans were available for nine patients. One was normal, as was the PET scan. Diffuse cortical hypoperfusion was present on the other eight SPECT scans. The PET findings were quite divergent in these cases: three were normal, three had bitemporal hypometabolism, one had hypometabolism only in the basal ganglia, and one had global hypometabolism. Thus, PET (cerebral metabolism) and SPECT (cerebral blood flow) did not provide identical information.
Late Disseminated Stage
The diverse clinical presentations that have been reported for Lyme disease create a diagnostic challenge. Multiple case reports document the later manifestations of untreated Lyme disease. Brainstem arteritis or cerebral vasculitis is not uncommon in
Borrelia positive patients. Various presentations have included Avellis syndrome (paralysis of soft palate and vocal cords unilaterally and loss of pain/temperature sensation contralaterally) and transient ischemic attacks intermittently over a 2 year period due to middle cerebral artery vasculitis.
23,
24 A presentation similar to supranuclear palsy (ataxic gait, inertia, memory disturbances) has also been reported.
25 In this case both structural and functional imaging were obtained prior to the correct diagnosis. Structural imaging (MRI and computed tomography [CT]) performed approximately 6 months after symptom onset showed only mild brain atrophy, and functional imaging (SPECT) was normal, leading to a diagnosis of spinocerebellar degeneration. A repeat SPECT scan 6 months later showed diffuse hypoperfusion in cerebral but not cerebellar cortex, bringing that diagnosis into question. Follow-up studies resulted in the diagnosis of Lyme neuroborreliosis.
More rarely, significant dementia or death has been documented. One such case included a previously healthy patient who presented with the classic rash, musculoskeletal pain, and joint swelling.
26 Despite multiple courses of antibiotics, he developed parkinsonian symptoms, eventual striatonigral degeneration, and death. The autopsy findings included mild atrophy of the basal ganglia, depigmentation of the substantia nigra, atrophy of the cerebellum, overall extensive neuronal loss, astrogliosis in the striatum, and substantia nigra. There were no Lewy bodies identified. Although multisystem atrophy commonly occurs in the absence of Lyme disease, it is worthy of note that the patient was very healthy with no signs of parkinsonism until the Lyme disease. He had repeated positive tests for the spirochetes. The authors of this article reviewed the few autopsy case reports in the literature and found no others with basal ganglia findings, but found commonly microgliosis, inflammation, vasculitis, and infarcts—primarily of the cortex, cerebellum, thalamus, and spinal cord.
A multitude of more specific neuropsychiatric symptoms have been reported in the literature resulting from Lyme disease. Some of these studies do not clearly distinguish between patients with untreated Lyme disease and patients with posttreatment residual symptoms. One summary of this literature notes that depression, decreased concentration, memory, and sleep disturbances occur early. With late stages, there are findings of significant worsening mood disorders, psychotic markers, panic attacks, severe dementias, personality changes, catatonia, and mania.
27Only a few studies have utilized functional imaging in patients with late stage Lyme disease and neuropsychiatric symptoms. One prospective study specifically selected patients with possible Lyme encephalopathy based upon the presence of neuropsychiatric symptoms (present for 2 months to 12 years).
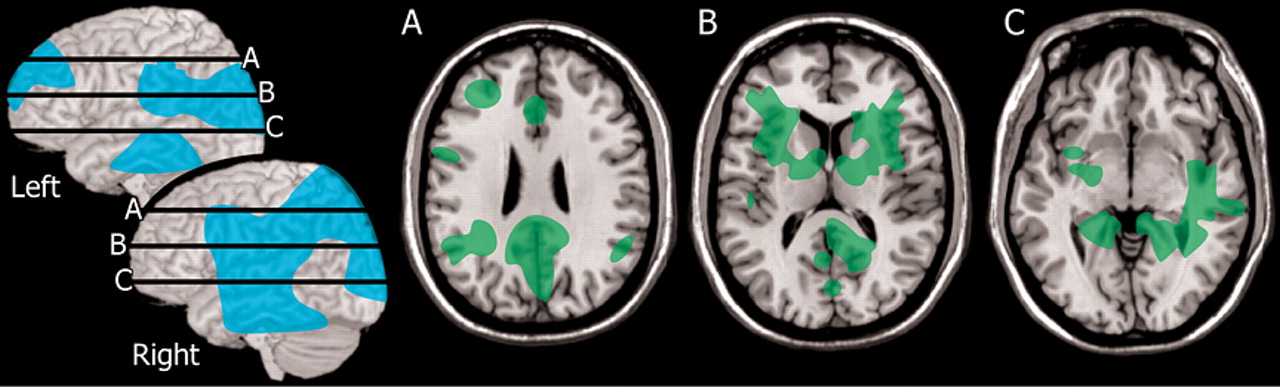
3 Neuropsychological testing, MRI, and SPECT were obtained from both the patients and a group of normal healthy individuals. More than half the patient group (13/22) were confirmed to have Lyme encephalopathy. Most of these (11/13) had normal MRI. Two had multiple areas of white matter abnormality. All (13/13) had multiple areas of hypoperfusion on SPECT, with both cortical and subcortical involvement. Group analysis indicated that the perfusion deficits clustered in lateral and medial frontal and medial temporal cortices, fronto-temporal white matter, and basal ganglia (
Figure 3 ). A 1-month course of antibiotic treatment was initiated. Most (11/13) of the Lyme encephalopathy group showed clear improvements in neuropsychiatric symptoms beginning 1–3 months after treatment. All had improved cerebral perfusion on repeat SPECT scans acquired at 6 months after treatment. One caveat on this study, however, is that only 2/13 had no previous antibiotic therapy and 5/13 had less than the recommended course. Thus, this group may include both late stage Lyme disease and post-treatment Lyme disease. A case study illustrates the diagnostic challenges.
28 The patient in this case study was initially diagnosed with anxiety and dysthymic disorder. Medication provided partial remission of anxiety symptoms, but did not alter the symptoms of depression, fatigue, or malaise. In addition, the patient’s arthritis symptoms continued to worsen. The patient was referred to internal medicine for evaluation of possible Lyme disease, which was confirmed. SPECT obtained at this time showed hypoperfusion of both temporal lobes. Antibiotic treatment resulted in gradual remission of symptoms. The authors of this case report noted the importance of considering the possibility of an infectious disease, particularly in the absence of any family history of psychiatric illness.
Chronic (Posttreatment) Lyme Disease
There is considerable controversy related to the possible existence of a chronic form of Lyme disease, and to the appropriate treatment approaches for symptoms that may remain following conventional antibiotic therapy. A recent “Point” and “Counterpoint” provides an overview for the interested reader.
29,
30 Commonly attributable symptoms to chronic Lyme disease have included long-standing fatigue, chronic musculoskeletal pain, and subjective cognitive slowing. Some authors feel strongly that chronic Lyme disease symptoms are coincidental to the initial infection, brought on by stress of illness, or a result of an inflammatory state.
6,
31,
32 Others feel that the evidence is sufficient to cite
Borrelia as a causative factor. A recent meta-analysis of reported late stage or post-treatment Lyme symptoms examined a total of 504 patients and 530 controls.
33 Identified symptoms for review included fatigue, musculoskeletal pain (swollen joints, muscle or joint pain, muscle aches), and cognitive deficits (memory, concentration, word finding, formulation of ideas/thoughts, judgment, and naming of objects). The authors concluded that fatigue, all three of the musculosketetal pain symptoms, and decreased memory, poor concentration, difficulties in formulating, ideas and difficulty in word finding were significantly more prominent in posttreatment Lyme disease patients than in controls. Challenges in interpreting these data include the inclusion of patients in various stages of infection/treatment, wide range of ages (adult and childhood intermixed data), and different levels of objective testing. The authors concluded, however, that after accounting for these challenges the data remain strong and significant that the above symptoms are more prominent in posttreatment Lyme disease patients. Of note, the above symptom patterns differ from those of chronic fatigue, fibromyalgia, and major depressive disorder. The interested reader is referred to the meta-analysis.
33Most reports of structural imaging in the posttreatment stage are similar to what has been reported during the early disseminated stage (see section “Early Localized and Early Disseminated Stages”).
34,
35 One study compared neuroimaging results between patients with focal (4/27) versus nonfocal (23/27) neurological deficits.
34 Patients with focal symptoms (e.g., cerebellar ataxia, sensory-motor deficits) had more widespread lesions in the white matter and a relapsing/remitting course similar to multiple sclerosis. Many (12/23) of the patients with nonfocal symptoms (e.g., fatigue, memory, depression) had normal MRI. A similar number (10/23) had punctate lesions in the white matter. Only one had multiple periventricular and subcortical lesions. Magnetization transfer imaging was obtained in the final eight patients with nonfocal symptoms. There were no differences between patients and normal healthy individuals in magnetization transfer ratio histograms. This is quite similar to the findings in the earlier stages of this disease (see section “Early Localized and Early Disseminated Stages”). The authors of this study note that this finding suggests that there is relatively little structural damage present.
Regional cerebral blood flow (rCBF) has been evaluated in a group of posttreatment Lyme disease (minimum 4 weeks of antibiotic therapy) patients utilizing xenon-133 and an external sensor array.
2 The major differences between the patient group and normal healthy individuals were in white matter perfusion. Patients had changes in the slower clearing compartment which is the white matter. Relatively lower perfusion was present in posterior temporal and parietal white matter (
Figure 3 ). The authors emphasized the potential for pathology in the white matter to disrupt processing within the cortical-subcortical networks that are critical for cognitive and emotional functioning.