P osttraumatic stress disorder (PTSD) is a common neuropsychiatric disorder with an estimated lifetime prevalence of 7.8% among adult Americans.
1 The presence of cognitive dysfunction associated with these patients has been documented in numerous studies, with reports now describing a variety of cognitive deficits associated with PTSD, including attentional deficits, memory impairments, and dysexecutive syndromes.
2 –
4 While there are multiple studies of cognitive dysfunction, few consistent cognitive deficits have been reported across these studies. In addition, it is unclear if the reported cognitive deficits represent pretrauma risk factors, are related to developing PTSD, or are a combination of both.
There are several plausible reasons for inconsistency in the identified cognitive dysfunctions. First, the studied PTSD populations can have confounding comorbidities, each with its own associated cognitive dysfunctions. The most common comorbidities include, but are not limited to, chronic alcohol and substance abuse, previous head injuries, and the presence of other psychiatric conditions. Alcohol abuse has been associated with impaired executive function,
5,
6 visuospatial processing, working memory,
7 and/or anterograde and retrograde memory, all of which could falsely be attributed to PTSD if a study population has significant alcohol use. In addition, Vietnam-era veterans have been reported to “self-medicate” with alcohol, marijuana, heroin, and benzodiazepines to ease the PTSD symptoms.
8,
9 Each of these substances has associated cognitive deficits; however, inconsistent and intermittent use of each substance in this population is typical, resulting in either those who have variable cognitive impairments or those who fluctuate in their degree of drug use.
10 Closed or open head injury with loss of consciousness has been associated with deceleration-acceleration injuries, diffuse axonal injury, hemorrhage/contusion, infarction, and focal traumatic lesions in the case of open head injury; each could lead to a different cognitive dysfunction.
11 –
13 Head injury is common among combat veterans with PTSD, and those with a history of head injury report higher levels of PTSD symptoms than those without a history of head injury.
14The presence of comorbid psychiatric diagnoses appears to have a large impact on cognitive function as well. Brady et al.
15 showed that a substantial number of PTSD patients have comorbid psychiatric diagnoses including substance abuse, depression, and anxiety disorders. Major depressive disorder has been associated with cognitive impairments,
16 and since a current or lifetime history of major depression may be present in a majority of patients with combat-related PTSD,
17 identifying the cognitive dysfunction associated with PTSD would have to account for these depression-related impairments.
Another factor that has obscured identifying the cognitive dysfunctions specifically associated with PTSD is determining whether deficits existed prior to, or developed secondary to, PTSD. Two key examples of this are IQ and memory. Vasterling et al.
18 suggested that after a comparable traumatic experience, one of the differences between those who acquire PTSD and those who do not is IQ—those with lower relative IQs appear to be at increased risk of developing PTSD. Brandes et al.
2 tested individuals within 10 days after a traumatic event, and those meeting criteria for PTSD had lower IQs, suggesting that IQ differences between subjects with and without PTSD either were premorbid in nature or occur very early in the course of PTSD. There are other factors that need to be considered before concluding that lower IQ is a predisposing condition for the development of PTSD. Sutker et al.
19 studied former Korean and World War II prisoners of war (POWs) and evaluated cognitive performance by groups (defined by weight losses of greater than 35% of premorbid weight, or less than or equal to 35%) and non-POW combat veterans. Percent weight loss is considered a physical marker of stress in prisoners, particularly if the prisoners were not starved. Sutker et al.
19 found that POWs with significant weight loss performed more poorly than combat veterans on subtests of the WAIS and Wechsler Memory Scale (WMS), suggesting that the severity of stress reflected by trauma-induced weight loss played a role in WAIS performance, making low WAIS IQ less likely a premorbid condition.
It is unclear which of the cognitive impairments reported in PTSD are genuinely related to developing PTSD, pretrauma risk factors, or both. The presence of comorbidities with their own associated cognitive deficits in those with PTSD, not all of which are delineated in each study, left interpretation of the previous findings difficult. In order to minimize these difficulties in data interpretation, we studied a group of former World War II POWs with comparable traumatic experiences, yet minimal comorbidities, and compared cognition in those who developed PTSD with those who did not.
METHOD
Subjects
A total of 25 male former POW subjects participated in the study. The Human Use Committee of the University of Arkansas for Medical Sciences approved the research protocol, and written informed consent was obtained from all subjects. All POW subjects both had combat experience and had been held captive during either World War II or the Korean War. Eight of the 10 former POWs with PTSD were German POWs and two were Japanese POWs. Seven of the 10 former POWs without PTSD were German POWs, two were Japanese POWs, and one was a Korean POW. All study subjects were carefully selected using stringent exclusion and inclusion criteria. All subjects were right-handed, had no history of traumatic brain injury with loss of consciousness, had no history of neurological impairment or degenerative neurological illness, did not meet criteria for either current or lifetime alcohol dependence or substance abuse, and had Mini-Mental State Exam (MMSE) scores of 26 or higher. All research subjects volunteered to complete the study without any compensation.
The subjects were recruited through a POW outreach program initiated by the Central Arkansas Veteran’s Health Care System. Only four subjects had a history of any previous psychiatric treatment; all four were POWs with PTSD. No study subject had a history of psychiatric hospitalization, alcohol or substance abuse treatment, or history of suicide attempt. The presence or absence of PTSD was ascertained with the use of the Clinician-Administered PTSD Scale (CAPS-2) for all subjects.
20 CAPS-2 scores were tallied for all subjects in terms of both total score and symptom cluster scores (re-experiencing, avoidance, and arousal). With the CAPS-2, the more conservative “rule of 4” was used
21 —i.e., the frequency and severity scores needed to meet symptom criteria had to add to a minimum of four. The Structured Clinical Interview for DSM-IV
22 was also administered to determine current and lifetime psychiatric diagnoses. Subjects were initially interviewed by a board-certified psychiatrist (TWF or TK) and their medical histories were also reviewed (LB). Military medical treatment files were reviewed to ascertain individual lengths of confinement and pre- and postconfinement body weights (LB). Based on diagnostic interview findings, POW subjects were divided into three groups: POWs with no PTSD or psychiatric diagnoses; POWs with PTSD
and psychiatric comorbidities (depression, panic disorder, phobia, generalized anxiety disorder); and POWs with PTSD only.
Neuropsychological Tests
The neuropsychological battery of tests for this population included the following:
•
Mini-Mental State Exam
23•
North American Adult Reading Test (NAART)—used as an estimate of IQ
24•
Controlled Oral Word Association Test
27,
28•
Digit span forward and backward (WAIS-III)
31•
Rey Auditory Verbal Learning Test
32•
Logical memory subtest of the WMS-R
33•
Warrington Recognition Memory Test (Faces)
34•
Judgment of line orientation
35•
Symbol Digit Modalities Test
36•
Geriatric Depression Scale
37,
38Procedures
The patients were tested in one of two dedicated research neuropsychological testing rooms with door insulation for noise reduction, comfortable seating, a testing table, no intercom or external speakers, and signs to designate that testing was in session. Testing was performed by trained neuropsychological testing technicians. The testing was performed in single sessions totaling approximately 80–90 minutes. The technician recorded all subject responses and scored them after the testing sessions. These responses were entered into a spreadsheet for further data analysis.
RESULTS
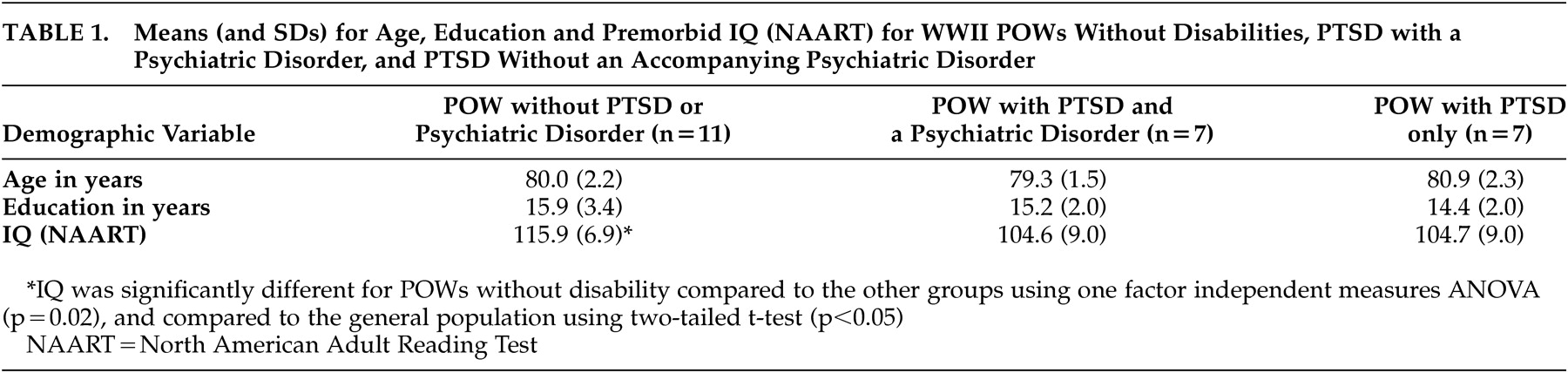
The data were entered into SPSS statistical analysis package (SPSS Inc., Chicago). The initial cross-sectional analyses across groups revealed several key findings (
Table 1 ), including the absence of significant group differences in age or education. A one-factor independent measures analysis of variance (ANOVA) on the North American Adult Reading Test IQ measure, grouped by POW diagnosis (no PTSD, PTSD only, PTSD + psychiatric comorbidities), was significant (F=4.59, df=2, 18, p=0.02). Post hoc t tests comparing groups to the population at large revealed a significant difference of IQ score of POWs without PTSD (t=2.99, df=7, p<0.05) and no significant findings with either PTSD group.
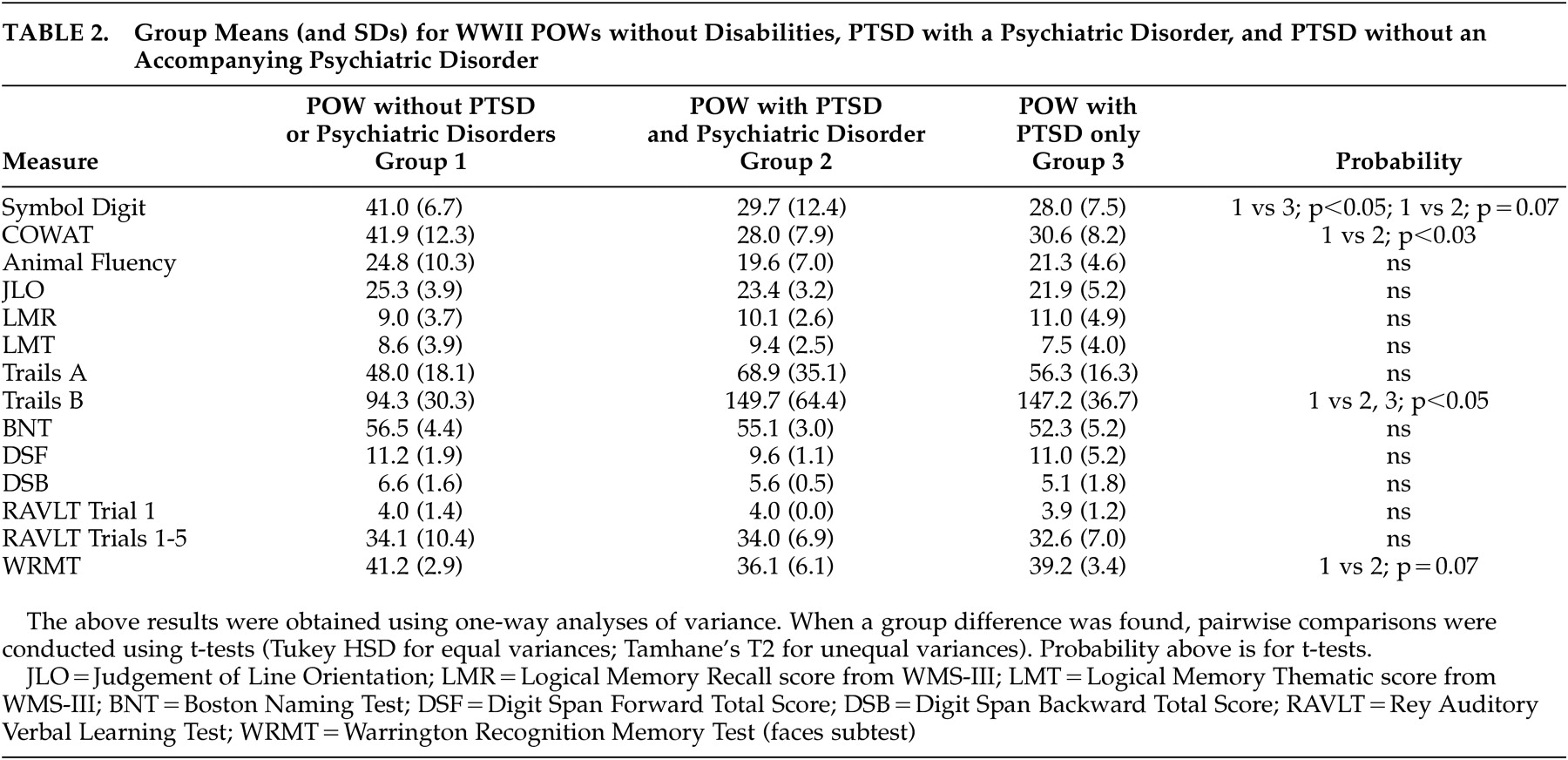
One-way ANOVA with all three groups for the neuropsychological test data revealed a significant group difference on Symbol Digit Modalities Test performance. Group performance was significantly less for those with PTSD only and demonstrated a trend toward a difference for the group with PTSD+psychiatric comorbidities (
Table 2 ).
Phonemic fluency (as measured by results on the Controlled Oral Word Association Test) was significantly less for the group with PTSD+psychiatric comorbidities for the total fluency score. It is of interest that animal fluency, more a measure of semantic processing than fluency, was not impaired for these groups (
Table 2 ).
Trails B performance was significantly slower for both the PTSD+psychiatric comorbidities and PTSD only groups compared to those without PTSD (
Table 2 ). Trails A performance was not different between any of the three groups.
There was a trend toward significance of recognition memory for faces in the PTSD + psychiatric comorbidities group (
Table 2 ).
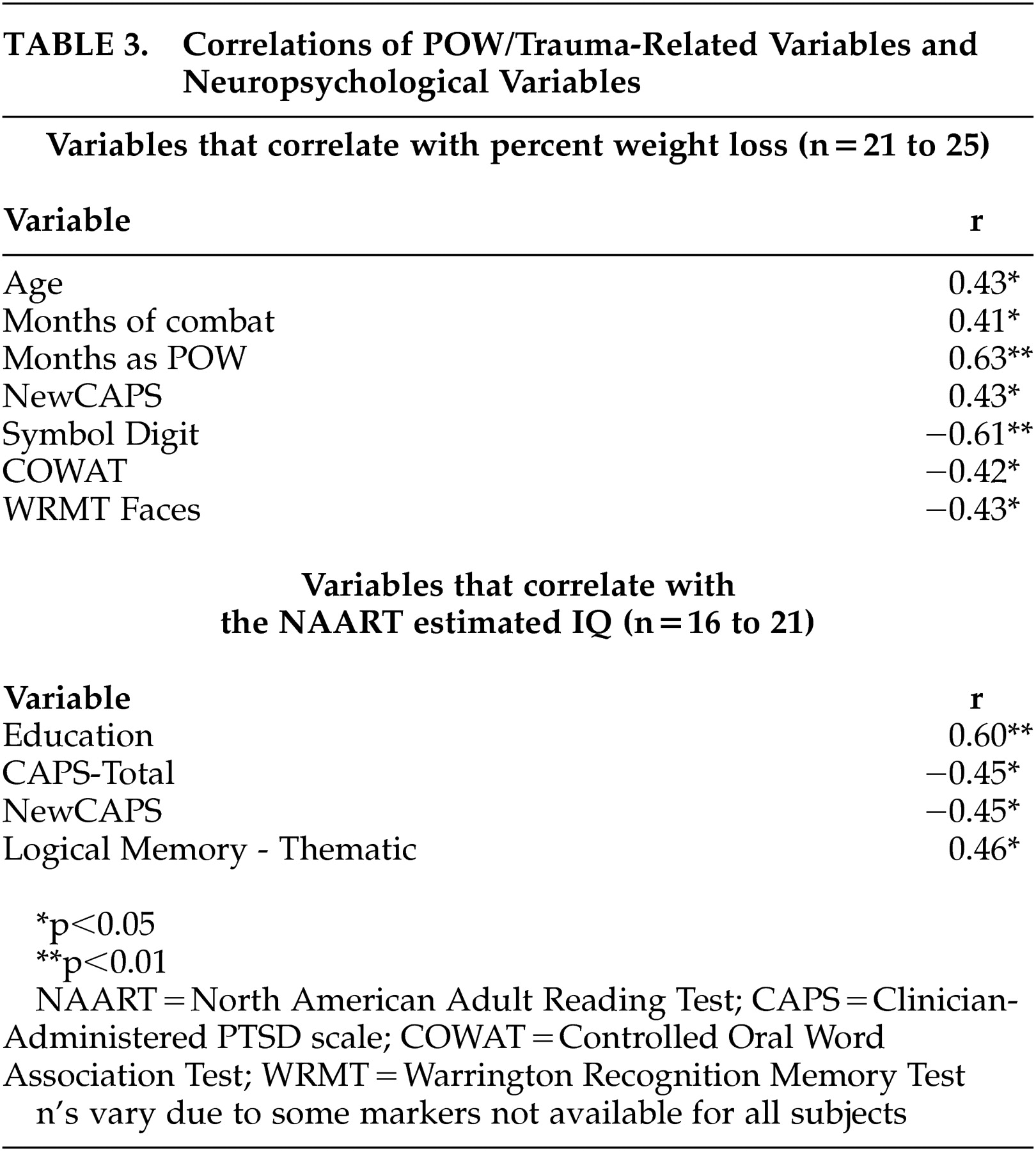
Correlations were assessed for variables associated with trauma, such as percent weight loss to pre-POW weight, number of months in combat, number of months as a POW, and total score on the Clinician-Administered PTSD Scale, as well as neuropsychological performance (see
Table 3 ). Percent weight loss correlated significantly with age, months of combat, months as a POW, total score on the Clinician-Administered PTSD Scale, as well as the neuropsychological variables of Symbol Digit, phonemic fluency (Controlled Oral Word Association Test), and recognition memory for faces. It is important to note that North American Adult Reading Test IQ
did not correlate with the markers of trauma (percent weight loss, months of combat, months as a POW, and total score on the Clinician-Administered PTSD Scale). A logistic regression analysis revealed that 29% of the probability of a diagnosis of PTSD could be accounted for by an individual’s IQ estimate (p<0.02). If percent weight loss during POW confinement is included as a variable in the logistic regression, then IQ estimate and percent weight loss together account for 40% of the variance of the probability of a PTSD diagnosis ( N=21; χ
2 =11.159, p<0.004, df=17).
DISCUSSION
As noted, it has been difficult to determine if there are specific cognitive dysfunctions associated with PTSD, and if so, whether these were precursors to, and/or are associated with, developing PTSD. This group of POWs provides a unique study of individuals with PTSD having minimal confounds and comorbidities. Additionally, this group of subjects was clearly exposed to severe trauma during early adulthood and had documented objective measurements of this trauma (e.g., weight loss, time of imprisonment) recorded at that time.
A major finding of the study was that IQ was significantly higher in those who did not develop PTSD compared to those who did. This was the case even when controlling for factors such as weight loss, time of imprisonment, and time as a POW. Given that the comparison group in this study was veterans who experienced comparable trauma, these differences in IQ cannot be attributed to the trauma alone. Since subjects with a history of head injury were excluded, head injury could not possibly account for such a difference. The most compelling finding was that premorbid higher IQ was protective against developing PTSD. While the other cognitive deficits that were detected had a significant association with markers of the traumatic event, IQ performance did not correlate with any of these factors: percent weight loss, months of combat, or months as a POW. Thus, the primary finding that higher IQ may protect people who experience a trauma from developing PTSD appears to be unrelated to the degree of trauma.
Symbol Digit, a marker of visual scanning and psychomotor speed,
39 and Trails B appear to be associated with PTSD in general, regardless of the presence or absence of psychiatric comorbidities (Symbol Digit significant for POWs with PTSD only and a trend for POWs with PTSD+ psychiatric comorbidities; Trails B significant for both). Psychomotor speed is a generalized, nonspecific cognitive impairment that does not necessarily imply pathologic changes in a focal anatomic structure, and it is unclear from the proposed models of pathogenesis in PTSD how this psychomotor dysfunction would develop. However, unlike IQ, this deficit is significantly correlated with factors relating to the traumatic event (e.g., percent weight loss) and is thus unlikely to be a premorbid or predisposing condition to developing PTSD. A relative decrement in performance on Trails B without a corresponding decrement for Trails A was noted in both PTSD groups. Isolated Trails B without Trails A decrements suggest that the psychomotor component present in both tasks is not likely to be the etiology of the decreased performance, but the source is the additional frontal lobe component of processing alternating sequences required in Trails B.
40,
41 While these two frontal lobe functions appear affected in PTSD, several other frontal lobe tests administered (digit span backward, category fluency) remain unaffected, further demonstrating the selectivity in frontal lobe disruption in PTSD.
4 The anatomic localization associated with Trails B performance has not been clearly identified in general and thus cannot be correlated to specific frontal regions in PTSD.
There are deficits associated only with those POWs with PTSD + psychiatric comorbidities and not with just PTSD alone. These are focal cognitive dysfunction in phonemic fluency and a trend toward a decrement in recognition memory for faces. While phonemic fluency is decreased for this group, it is of interest that animal fluency, more indicative of semantic processing compared to phonemic fluency, is not impaired in this group. In functional imaging studies, phonemic fluency has been associated with the left inferior frontal gyrus, while semantic fluency is not,
42 suggesting this frontal region may be affected in this PTSD group. Neuroimaging studies have implied that there is dysfunction in the inferior frontal region in PTSD, with reports of decreased frontal lobe volume in PTSD relative to comparison subjects
43 and decreased activation in functional imaging studies in Broca’s area during trauma evoked memories in PTSD patients.
44 Delineation of the anatomic regions and pathologic changes associated with the cognitive dysfunction in PTSD should provide further insights into the pathogenesis of these deficits.
The trend toward significance of recognition memory for faces is noted here only because of several previous reports of medial temporal structural abnormalities with PTSD. Performance on recognition memory for faces has been associated with right temporal lobe pathology,
34 and PTSD neuroimaging studies have shown asymmetrically smaller right hippocampi
45 —this finding is one of the clearest links between function and pathology in PTSD. It is notable that this potential difference is present in the PTSD + comorbid psychiatric pathology group and not in the PTSD only group, suggesting that PTSD alone may not be a sufficient condition for this level of relative impairment.
There ostensibly appear to be two groups of cognitive markers associated with PTSD in this population—those premorbid markers unrelated to the trauma (relatively high IQ) and those associated primarily with developing PTSD. Of those cognitive dysfunctions that appear to be associated with developing PTSD, the most robust quantitative marker of trauma correlated with them is percent weight loss during POW confinement, which in these subjects is thought to represent an individual’s response to the stress of imprisonment.
46 The focal cognitive dysfunctions associated with PTSD in this population (psychomotor speed with Symbol Digit, phonemic fluency as measured by Controlled Oral Word Association, and recognition memory for faces assessed with the Recognition Memory Test) were significantly correlated with percent weight loss, further suggesting that these are unlikely to be premorbid risk factors for developing PTSD.
The cognitive deficits reported here have been reported previously with PTSD; however, not all of these studies specified whether the patients had PTSD alone or had other psychiatric comorbidities. Our findings suggest that PTSD alone may not be sufficient to result in some of these deficits but that they occur only when the subjects have PTSD and comorbid psychiatric diseases.
These findings may also have significant implications for aging, specifically the finding of higher IQ levels in those who do not develop PTSD. Relatively higher IQ status has been suggestive to be protective against developing progressive cognitive decline with aging,
47 including developing dementia.
48,
49 One study has directly assessed aging in PTSD among Holocaust survivors and found that those with PTSD had a lower IQ than those without PTSD and elderly control subjects not exposed to the Holocaust. More important, explicit recall was impaired for the PTSD group, with 36% (N=31) of these patients impaired to a level of clinical significance. Older age was significantly associated with poorer memory performance in the PTSD groups, but not in the two control groups, with accelerated memory decline one of the more likely explanations for the significantly greater association of older age with poorer explicit memory in the PTSD groups.
50 These results from Holocaust survivors and the results of this study would suggest that older survivors with PTSD (particularly with relatively lower IQ and prevalence of comorbid psychiatric illnesses) may be at risk for developing progression of cognitive dysfunction with aging. The most likely group would be those with existing memory deficits. The present group of Vietnam-era veterans with PTSD should be monitored for progression of accelerated cognitive decline with aging. In addition, there have been reports of an increase in re-experiencing PTSD symptoms concurrent with the development of dementia in several patients (i.e., via loss of frontal lobe suppression of these symptoms as aging diminishes the effectiveness of frontal lobe function).
51 Further studies assessing these factors prospectively in the aging Vietnam-era veterans with PTSD are indicated to better address this issue in terms of preventative, diagnostic, and treatment options. The present study did not address these factors in that none of the subjects reported any subjective complaints nor were any showing signs of dementia. We acknowledge that this POW group is select and represents those individuals who survived until later life without cognitive complaints. These individuals were chosen as they represent a group unlikely to have cognitive difficulties and thus provide the clearest evidence if cognitive deficits existed with PTSD.
There are several limitations to the present study. The number of subjects is relatively small and had a higher education level than the general population. They are also a selective group of POWs who lived into their eighth decade and are being assessed years after the traumatic event occurred. While these issues could limit the ability to generalize the findings, this population without a significant history of alcohol and/or substance abuse and including a group of veterans with PTSD only render this study population uniquely informative.
Overall, the present study provides a well-defined cohort with documented trauma and minimal comorbidities to aid in delineating cognitive dysfunction that is most likely associated with PTSD itself. Our findings suggest that higher IQ is a premorbid protective factor providing resilience from developing PTSD after a traumatic experience. Compared to those POWs who experienced similar traumas and did not develop PTSD, those who developed PTSD (with either psychiatric comorbidities or PTSD alone) had deficits in a selective test of frontal lobe function (Trails B) and visual scanning and psychomotor speed (Symbol Digit). Those with PTSD and psychiatric comorbidities had additional deficits in another selective test of frontal lobe function (phonemic fluency) and a trend toward a decrement in recognition memory for faces. These findings have important implications for other patient populations (e.g., child physical abuse, sexual abuse, involvement in a life threatening accident, natural disaster, or witness of another being badly injured or killed) with a high prevalence of PTSD.
Acknowledgments
We would like to thank Cynthia Wills and Dena Davenport for testing the patients and all of the Veterans who graciously gave of their time to participate in this study.