A pathy is a complex neurobehavioral syndrome that is characterized by a lack of motivation.
1,
2 More broadly, it is conceptualized as the absence of responsiveness to stimuli as demonstrated by a lack of self-initiated action, lack of concern, and emotional indifference.
1,
3,
4 Apathy is common in a number of clinical health conditions including major depression,
3,
5 schizophrenia,
6 Alzheimer’s disease and other dementias,
1,
7 –
11 traumatic brain injuries,
12 –
15 cerebrovascular disease,
15 –
17 and HIV/AIDS.
18 Apathy is associated with poor treatment outcome, poor compliance with treatment regimen, caregiver stress, decreased level of functioning across a number of health conditions,
12,
14,
15,
19 –
21 and is of both clinical and public health importance. This necessitates efforts to gain a better understanding of the pathogenesis of apathy.
Knowledge of the clinical and sociodemographic correlates of apathy will help to enhance our understanding of the pathogenesis of apathy, and conversely, data related to apathy will contribute to our understanding of important neuropsychiatric disorders. The literature has identified clinical factors that are associated with apathy. For example, in a study with 40 outpatient participants with Alzheimer’s disease, apathy was found to correlate highly with other behavioral problems and with insight.
9 Resnick and colleagues
20 found significant and positive associations between apathy and depression and significant and negative associations between apathy and cognitive functioning in a clinical sample of geriatric patients. The researchers also reported that apathy was a significant predictor of functional level following rehabilitation treatment for hip fracture, stroke, joint replacement, or amputation.
20 Levy et al.
22 studied 154 patients with Alzheimer’s disease, frontotemporal dementia, Parkinson’s disease, Huntington’s disease, and progressive supranuclear palsy and found significant differences between the groups in terms of apathy as measured by the apathy subscale of the Neuropsychiatric Inventory. The group observed apathy to be most common in progressive supranuclear palsy (77%), quite common in frontotemporal dementia (61%) and Alzheimer’s disease (43%), and less common in Huntington’s disease and Parkinson’s disease (21% and 5%, respectively).
22 In addition to apathy, the dementia groups also differed on important variables such as age, cognitive function, and dysphoria. Apathy did not correlate with depression for the entire sample, and the researchers concluded that apathy and depression were distinct behavioral syndromes in these populations. Apathy, they explained, is quite common in some, but not all, neurological disorders.
22The present study adds to the current literature by examining the correlates of apathy with an array of clinical and sociodemographic factors. Multiple measures are used to ascertain the presence and levels of apathy to enable the examination of how the frequency of occurrence and correlates of apathy might differ across measures. Specific focus is placed on the physician’s diagnosis, the use of the Clinician and Informant versions of the Apathy Evaluation Scale (AES) using identified cutoff scores,
23 and the apathy subscale of the Neuropsychiatric Inventory. The AES is an 18-item scale that was developed by Marin and colleagues
1 to characterize and quantify apathy in dementia. In the development of the AES, apathy was operationalized as a psychological construct characterized by coexistent deficits in overt behavioral, cognitive, and emotional concomitants of goal-directed behavior, and included items that capture these domains.
1,
2It is important to note that prior to our examination of the clinical and sociodemographic correlates of apathy, we reassessed the factor structure and psychometric properties of the AES
23 based on our previous conclusion that there was a lack of studies reexamining these issues in order to provide supporting or refuting evidence relative to the original findings.
23 The Clinician and Informant versions of the AES were found to be comprised of two factors, “apathy” and “interest,” but the apathy factor accounted for more of the variance for the AES Clinician version (AES-C) than the interest factor, while the reverse was observed for the AES Informant version (AES-I).
23 The following are examples of items that loaded onto the apathy factor: “Getting things started/done on his or her own is important to him/her,” “S/he has motivation,” and “S/he gets things done during the day.” Examples of some of the items that loaded on the interest factor were: “S/he is interested in things,” “S/he is interested in learning new things,” and “S/he spends time doing things that interest her/him.” These two versions of the Apathy Evaluation Scale (AES-C and AES-I) were found to have fairly good to optimal performance. For the AES-C, the internal consistency was 0.86 and 0.90 for the “interest” and “apathy” factors, respectively, and based on the optimal cutoff score of 40.5, sensitivity was 85.7% and specificity was 58.0%.
23 The sensitivity and specificity of the AES-I using the optimal cutoff score of 41.5 were 92.9% and 56.6%, respectively, with internal consistencies of 0.88 and 0.99 for the “interest” and “apathy” factors, respectively.
23 The results of our previous study were used to guide the analyses in the present study.
The present study also used the psychiatrist’s clinical diagnosis of apathy as well as the scores on the AES-C, AES-I, and the Neuropsychiatric Inventory apathy subscale as methods of apathy assessment for the following reasons: the physician’s diagnosis is important for the treatment of apathy as well as for the dementias in which it is quite common; the AES was developed specifically to characterize and quantify levels of apathy in individuals ages 50 and older with and without dementia, and has been used consistently across studies on apathy;
1,
5,
13 –
15,
20 and the apathy subscale of the Neuropsychiatric Inventory is an alternate scale used across studies to measure apathy. Using these measures, we hypothesized that apathy would occur frequently in the dementias regardless of the methods used to assess its presence or level. However, based on our previous finding that the AES-I had optimal performance compared to the fairly good performance of the AES-C,
23 we hypothesized the frequency of apathy across the dementias would differ depending on the methods used in its ascertainment. Based on the different cutoff scores for the AES-I (41.5) and AES-C (40.5), the associations between the clinical and sociodemographic factors and apathy will differ slightly.
METHOD
Settings
Participants were drawn from the Behavioral Neurology Clinic at Baycrest Centre for Geriatric Care in Toronto, Ontario. This is an outpatient multidisciplinary clinic headed by the Behavioral Neurology Department. The team was comprised of clinicians in neurology, psychiatry, neuropsychology, communication disorders, occupational therapy, and social work. The clinic provides thorough diagnostic assessments and management of patients with suspected cognitive disorders. The referral sources were primary care physicians and specialists.
Participants
For a period of 2 years in the late 1990s, 170 patients with memory complaints were referred to and seen by a behavioral neurologist (MF) to ascertain dementia status. Of these, all patients in whom the presence of dementia was strongly suspected (N=121) were referred to psychiatry for assessment and management as indicated. The psychiatric evaluation included further testing for dementia by a neuropsychologist (MS) and assessment for the presence of neuropsychiatric disturbances, including apathy, depression, psychosis, and other behavioral changes. The sample primarily consisted of community-dwelling individuals still living in their own homes as well as a few nursing home residents. All were able to attend an outpatient assessment. The 121 participants who were referred for psychiatric assessments formed the study sample.
Psychiatric Assessment
The psychiatric assessments were comprised of two components. First, the presence of apathy and other neurobehavioral conditions were assessed by the clinical research assistants through both patient and caregiver/relative interviews using the structured inventories described in the following section. The data were then provided to the psychiatrist (RvR) and his resident, who incorporated this information into the psychiatric assessment. Second, the psychiatrist and his resident then completed unblinded clinical assessments for apathy, depression, psychosis, and other behavioral changes. Although privy to the information from the clinical research assistants, the psychiatrist’s diagnosis of apathy was based on Marin’s guidelines for diagnosing apathy.
2,
3 These diagnostic conclusions were then taken from the dictated consultation notes by the clinical research assistants.
The Clinical Inventories
The two versions of the Apathy Evaluation Scale
1 were utilized to measure the level of apathy. Our previous study that used the psychiatrists’ “unblinded” diagnosis of apathy as the “gold standard” reported cutoff scores of 40.5 and 41.5 for the Clinician and Informant versions of the AES, respectively.
23 Individuals scoring at or above these cutoff scores were characterized as apathetic, and those scoring below the cutoff scores were characterized as non-apathetic in separate analyses. The “apathy” and “interest” factors of the AES-I and AES-C observed in our previous study
23 were also examined as outcome variables in separate analyses in this study.
The Neuropsychiatric Inventory was also used to examine clinical correlates of apathy including delusions, hallucinations, agitation, depression, anxiety, elation, disinhibition, irritability, aberrant motor behavior, sleep behavior, and appetite behavior. The Neuropsychiatric Inventory was developed by Cummings et al.
19 to measure this range of behavioral disturbances and reportedly has good psychometric properties.
10,
24The presence or absence of depression was assessed by the Structured Clinical Interview for DSM-III-R (SCID)
25 This is a widely used semistructured interview for DSM diagnoses. Only the depression portion of the SCID was included in the assessment.
Functional status was initially assessed using the Physical Self-Maintenance Scale
26 for basic activities of daily living with a range of scores from 0 to 6. The Instrumental Activities of Daily Living Scale
26 measures more complex or instrumental activities of daily living than the Physical Self-Maintenance Scale, with scores ranging from 0 to 8. For both scales, higher values represent less disability. At a bit more than halfway through the period of data collection, the Disability Assessment for Dementia (internal consistency=0.96, interrater reliability=0.95, and test-retest reliability=0.96)
27 scale was selected to replace the Physical Self-Maintenance Scale and Instrumental Activities of Daily Living Scale. With the Disability Assessment for Dementia scale, caregivers were systematically asked 40 questions about the patients’ abilities to initiate and plan (i.e., intention), and organize and effectively perform (i.e., action) both basic (17 items) and instrumental activities of daily living (23 items). The scoring of the Disability Assessment for Dementia is based on a combination of the patient’s intention and action scores, with higher scores indicating less impairment.
27 The scores on both components of the Disability Assessment for Dementia (i.e., basic activities of daily living and the Instrumental Activities of Daily Living Scale) are converted to respective percentage scores, with higher scores being indicative of fewer disabilities in activities of daily living.
27 In this study, the scores on the Physical Self-Maintenance Scale and the Instrumental Activities of Daily Living Scale were converted to respective percentage scores in order to be compatible with the Disability Assessment for Dementia scoring. The percentage basic activities of daily living and Instrumental Activities of Daily Living Scale scores were used in the study.
The widely used Mini-Mental State Exam (MMSE)
28 was used to assess the cognitive functioning of the patient population. The scale reportedly has good psychometric properties.
28,
29Diagnosis of Dementia
Following the assessment of each participant by the behavioral neurologist (MF), the psychiatrist (RvR), and neuropsychologist (MS), as well as cerebral imaging that was ordered (CT, MRI, single photon emission computed tomography [SPECT]), the participant’s chart was reviewed to determine whether the individual met National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria
30 for possible or probable Alzheimer’s disease, the Consortium on Dementia with Lewy Bodies criteria
31 for dementia with Lewy bodies, the Lund and Manchester criteria for frontotemporal dementia
32 and/or the Hachinski criteria for vascular dementia.
33 Participants who met multiple diagnostic criteria received a diagnosis of mixed dementia.
Data Analysis
Combinations of descriptive and bivariate analyses were conducted to examine the study’s objectives. Student t tests, analysis of variance (ANOVA), chi-square analyses and Mann-Whitney tests were conducted where appropriate to describe the study sample and to compare individuals with and without apathy based on the identified cutoff score for the AES-I and AES-C from our previous study.
23 Multiple linear or logistic regression analyses (for continuous and categorical dependent variables, respectively) were utilized to determine the variables most predictive of apathy using an a priori statistical significance value of 0.05 or less. Bonferroni corrected p values were also calculated. All analyses were performed using SPSS for Windows version 12.0.
34RESULTS
Characteristics of the Participants
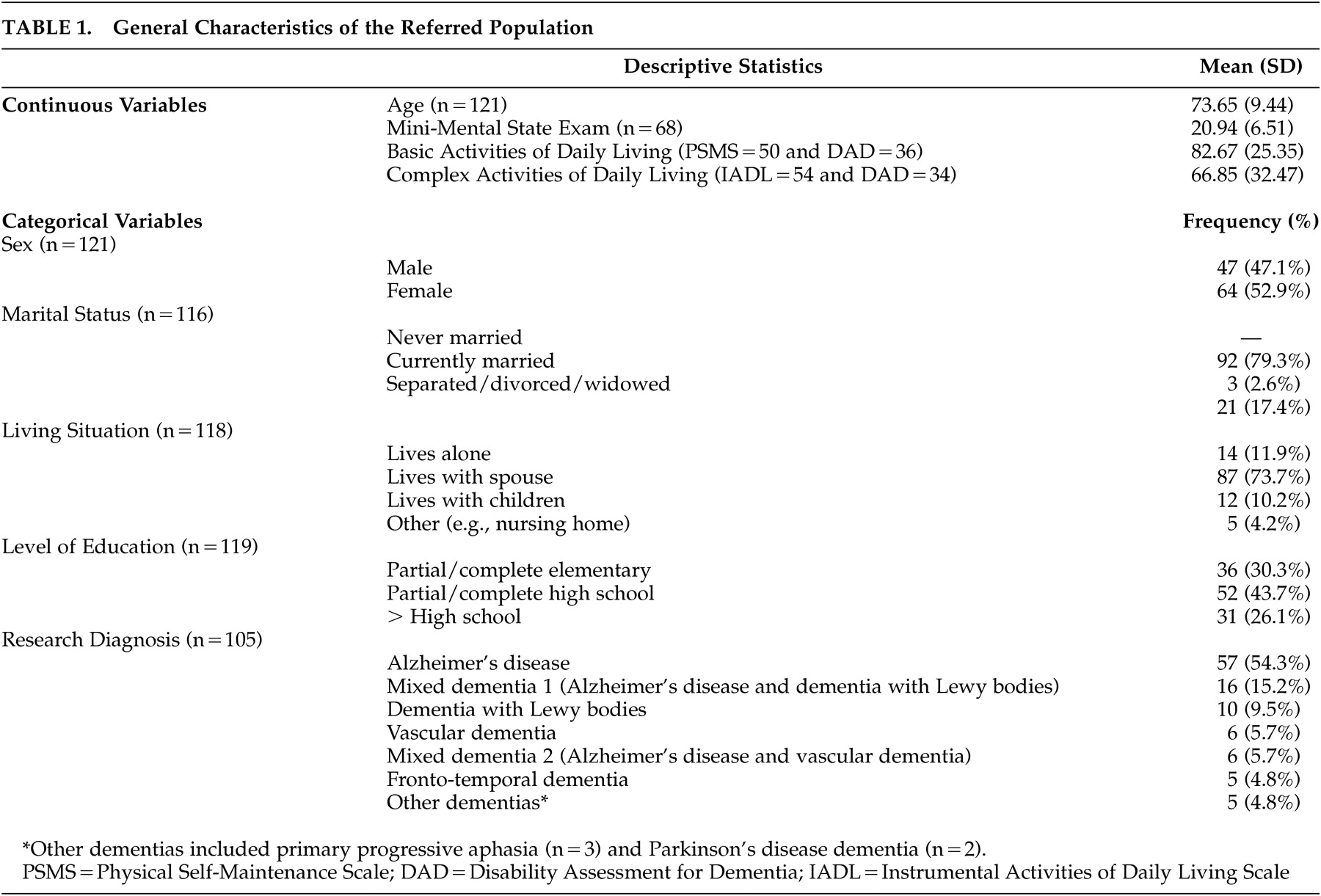
Table 1 summarizes the demographic and clinical characteristics of the 121 participants referred for psychiatric assessment. The age range of the study population was 48 to 91 years. The sample consisted of slightly more females than males (n=64; 52.9%) with most patients being married (n=92; 79.3%) and living with a spouse (n=87; 73.7%). Forty-four percent had a partial to complete high school education. On average, the MMSE score for the group was 20.9 (±6.5). The group scored higher in activities of basic self-care (mean=82.7±25.4) compared to instrumental activities of daily living (mean=66.9±32.5). Of the 105 participants for whom research diagnoses were ascertained, the majority met criteria for Alzheimer’s disease (54.3%). The remaining participants met diagnostic criteria as outlined in
Table 1 .
The Frequency and Correlates of Apathy in the Dementias
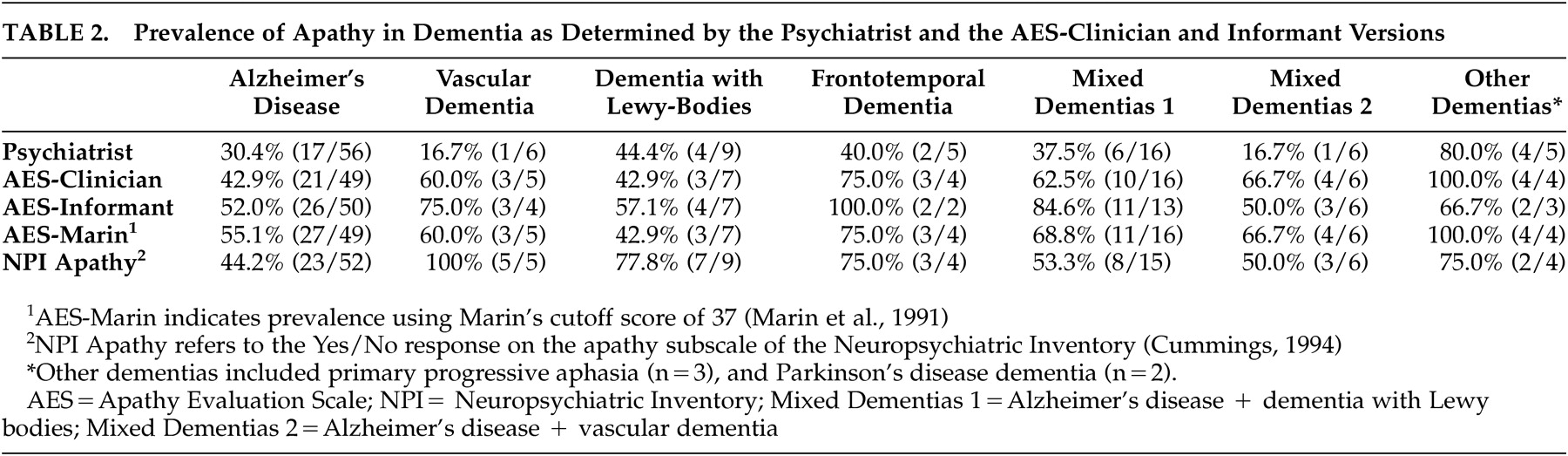
Table 2 illustrates that apathy is very prevalent across the dementias, regardless of the criteria used for its ascertainment. Small sample size for dementia groups other than Alzheimer’s disease precluded comparative analyses of apathy across the dementias. The frequency of occurrence of apathy in Alzheimer’s disease ranged from 30.4% to 61.2%, depending on the criteria used, and was consistently high in frontotemporal dementia (40.0%-100.0%) and other dementias (66.7%-100%).
Correlates of Apathy Using the Apathy Evaluation Scale-Informant version (AES-I)
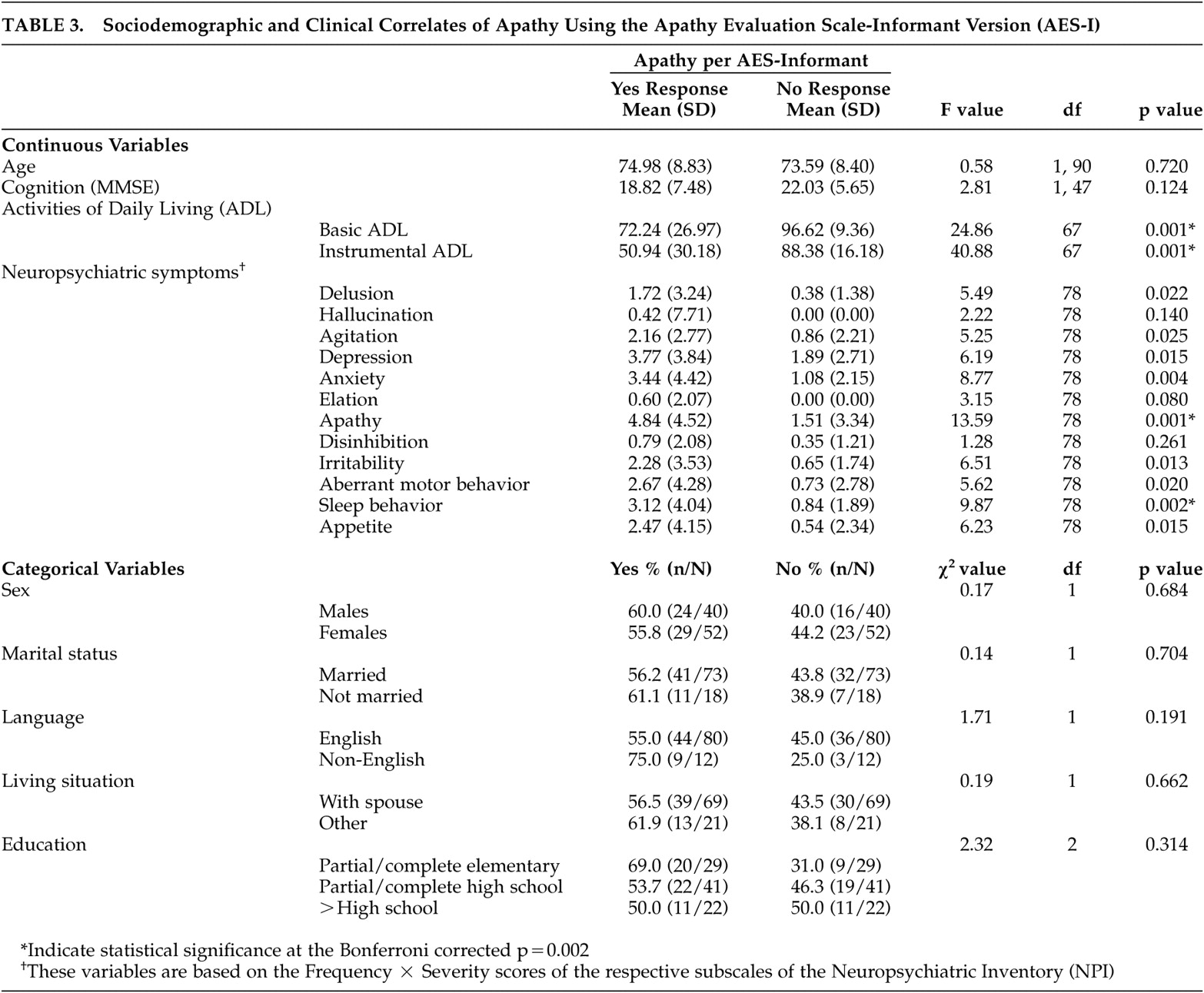
Table 3 outlines the sociodemographic and clinical correlates of apathy, using the cutoff score (41.5) for the Apathy Evaluation Scale-Informant version (AES-I). The bivariate analyses showed no differences in age, gender, marital status, living situation, language, education, and cognition between individuals who scored at or above the cutoff value for apathy observed for the AES-I (≥41.5 on the AES-I, apathetic) and individuals who scored below the cutoff (<41.5 on AES-I, nonapathetic). However, apathetic individuals were more likely to be impaired in basic self-care activities (F=24.86, df=1, 67, p=0.001) and instrumental activities of daily living (F=40.88, df=1, 67, p=0.001) compared to nonapathetic individuals. Similarly, compared to nonapathetic individuals, those with apathy were more likely to report delusions, agitation, depression, anxiety, irritability, aberrant motor behavior problems, and sleep and appetite disturbances. When Bonferroni’s correction was applied (alpha level p=0.002) most of the statistical significance disappeared. The notable exceptions were the differences between individuals with apathy and those without on basic self-care activities, instrumental activities of daily living, and sleep behavior disturbances.
Using the prior criterion of statistical significance of 0.05 or less for variable inclusion in the multivariate model, multivariate logistic regression with age, basic and instrumental activities of daily living, delusion, agitation, depression, anxiety, irritability, aberrant motor behavior, and sleep and appetite disturbances in the model showed that instrumental activities of daily living (odds ratio=0.92; 95% Confidence Interval [95% CI]=0.87, 0.99) and aberrant motor behavior (odds ratio=0.52; 95% CI=0.29, 0.94) had statistically significant relationships with apathy. That is, individuals with higher levels of functioning, as indicated by higher scores on the Instrumental Activities of Daily Living Scale, and more aberrant motor behavior problems were less likely to be apathetic using the cutoff score on the AES-I. There was a trend for individuals with apathy to have higher levels of irritability compared to nonapathetic individuals (odds ratio=1.87; 95% CI=0.97, 3.64, p=0.07). The sample size for this model was 64, but when the MMSE score was included in the model, it was reduced to 36. As such, the MMSE was excluded from the model.
When the scores on the “apathy” factor of the AES-I were correlated with the sociodemographic and clinical variables, the same pattern emerged as above. However, correlates of the “interest” factor differed slightly. Agitation, depression, sleep and appetite disturbances and basic and instrumental activities of daily living were significantly and directly correlated with impairment on the “interest” factor.
Correlates of Apathy Using the Apathy Evaluation Scale-Clinician Version (AES-C)
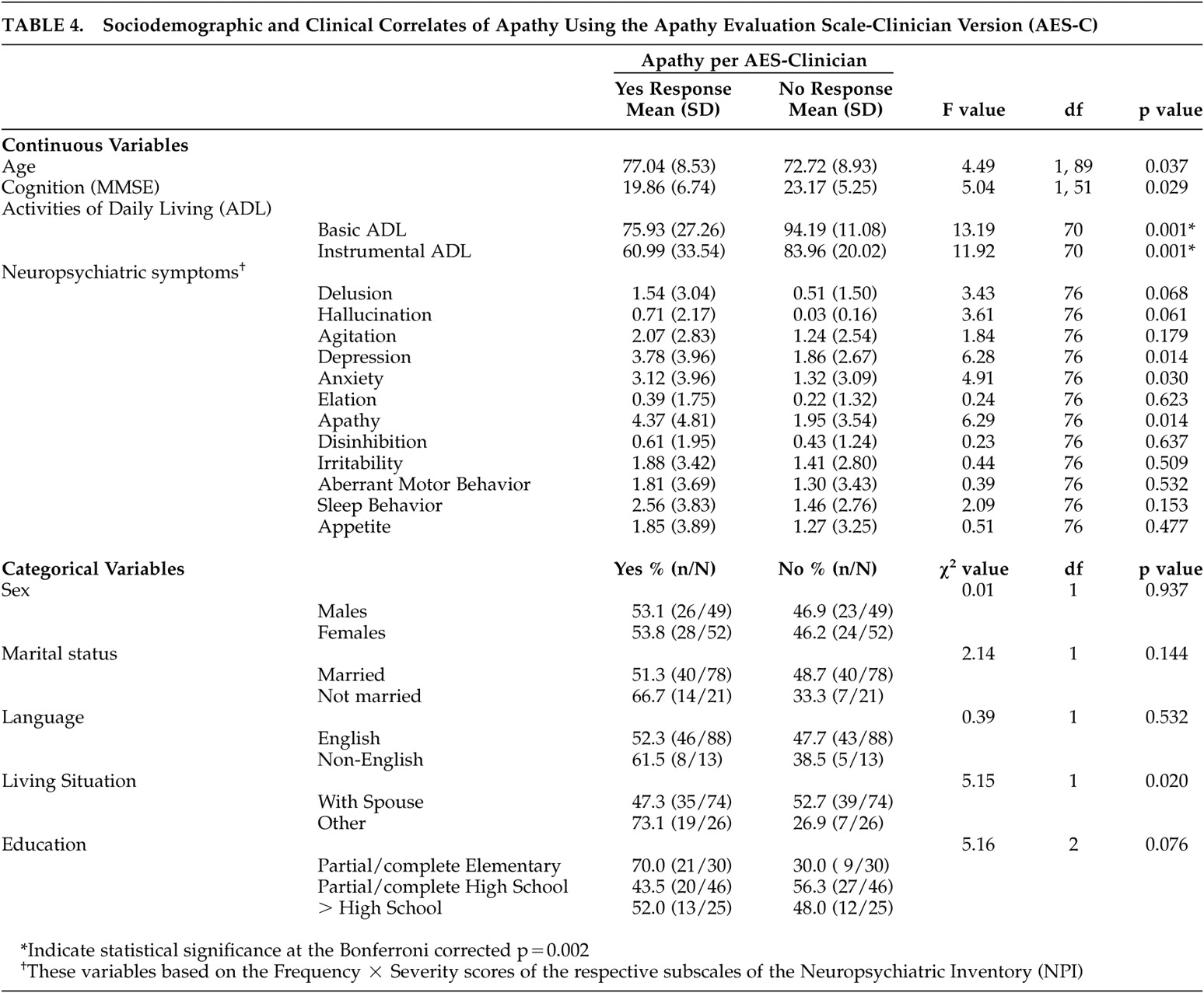
The results, using the cutoff score for the AES-C (40.5) to identify apathetic and nonapathetic individuals, revealed slightly different results from those observed with the AES-I (
Table 4 ). At the bivariate level, age, cognition (per the MMSE), living situation (with a spouse or not), basic and instrumental activities of daily living, depression and anxiety had statistically significant relationships with apathy. Upon application of Bonferroni’s correction, only basic and instrumental activities of daily living remained statistically significant. Further, multivariate logistic regression modeling, with age, living situation, basic and instrumental activities of daily living, depression, and anxiety in the model, revealed that only living situation had an independent and statistically significant relationship with apathy (odds ratio=4.56; 95% CI=1.12, 18.59, p<0.01). Individuals living in situations other than with a spouse were 4.56 times more likely to be apathetic than those living with a spouse. Again, low sample size for the MMSE scale resulted in cognitive functioning being excluded from the model.
At the bivariate level of analysis, correlates of the “apathy” factor of the AES-C were similar to those of apathy (yes compared with no) based on the cutoff score for the AES-C (40.5) (i.e., age [r=0.21, p<0.05], education [r=−0.23, p<0.05], and basic [r=−0.45, p<0.001] and complex [r=−0.40, p<0.001] activities of daily living), but also included the “interest” factor of the AES-C (r=0.65, p<0.001). When Bonferroni’s correction was used (p<0.002), the statistical significance disappeared for age and education. Multiple linear regression analyses with all five variables and using backward elimination showed that only basic activities of daily living (t=−3.93, p<0.001, β=−0.10) and the “interest” factor (t=6.08, p<0.001, β=−1.32) had independent and statistically significant relationships with the “apathy” factor of the AES-C.
With respect to the “interest” factor of the AES-C, age (r=0.28, p<0.01), living situation (r=0.20, p<0.05), education (r=−0.31, p<0.01), basic activities of daily living (r=−0.25, p<0.05), and the “apathy” factor (r=0.65, p<0.001) were identified as the sociodemographic and clinical correlates at the bivariate level of analysis. Only the apathy factor remained statistically significant when Bonferroni’s correction was used. At the multivariate level of analysis, only living situation and apathy had independent and statistically significant relationships with the interest factor of the AES-C.
DISCUSSION
The main finding of this study is the high frequency of apathy across the dementias. These results are consistent with previous reports of high prevalence of apathy across dementia illnesses.
21 A number of sociodemographic and clinical factors such as age, living situation, level of functioning, and neurobehavioral symptoms such as depression, delusion, agitation, and irritability were found to be correlated with apathy. Cognition was also found to be associated with apathy but only at the bivariate level of analysis and without Bonferroni’s correction. However, before further discussion of the findings, it is important to point out certain limitations of the study. There was a lengthy waiting list for the Behavioral Neurology Clinic, which might have led to a referral bias (e.g., there may have been a selection bias toward more diagnostically challenging or more difficult to manage cases being seen). In addition, of the 121 individuals who were referred to psychiatry, only 105 were seen and had a research diagnosis. Fifteen individuals failed to show up for their appointment. However, the “no shows” were not different from the study sample in terms of age, sex, marital status, living situation, or education. We were also unable to maintain blindness of the psychiatrist to the results of the AES, and other neurobehavioral measures, as these were clinical data. It is of course possible that these results would influence the psychiatrist in terms of their assessment of the presence or absence of apathy. Similarly, the AES-C and the other measures, including the SCID, Physical Self-Maintenance Scale, Instrumental Activities of Daily Living Scale, and the Disability Assessment for Dementia scale, were administered by the same clinical research assistant during the same interview session for each subject; results from one inventory may have influenced results from the other inventories. Missing data also affected our ability to conduct some relevant comparative analyses. For example, small numbers in some diagnostic categories prevented the investigation of the severity of apathy across diagnostic groups. Also, the cross-sectional nature of the data limits our ability to determine causation. Despite these limitations, this research has provided information related to the high prevalence of apathy in dementia and the sociodemographic and clinical correlates of apathy in dementia based on cutoff scores relevant to this population. These results are hypothesis-generating in that a number of relationships observed herein support the need for longitudinal studies to determine causation.
The results showed that apathy occurred frequently across the dementias. The psychiatrist had a higher threshold for diagnosing apathy compared to the other measures used to ascertain apathy status. The reasons for this difference were unclear as this issue was not assessed. However, this may point to the need for clearer clinical diagnostic criteria for apathy, an issue currently being addressed. The psychiatrist utilized Marin’s proposed diagnostic schema as a guideline for diagnosing apathy,
2,
3 and these criteria may have been more stringent than the criteria used in the apathy scales (AES-I, AES-C, and the apathy subscale of the Neuropsychiatric Inventory). Interrater agreement for the clinical diagnosis of apathy has not been established and needs to be.
The assessment of the clinical and sociodemographic correlates of apathy was conducted with a view to gain better understanding of the clinical relevance and pathogenesis of apathy in the dementias. From the available data, apathetic individuals were more likely to be older and functionally impaired at the bivariate level of analyses compared to nonapathetic individuals. These findings were consistent with previous studies on apathy using the AES or other measures of apathy.
1,
7 –
9,
12,
21,
35 –
37 Individuals with dementia and related behavioral problems including delusions, depression, agitation, anxiety, aberrant motor behavior, sleep and appetite disturbances, and having difficulties with instrumental activities of daily living were more likely to be described as having apathy by their informant. Since apathy has been linked to caregiver distress,
7,
20,
21 lack of compliance with treatment and poor level of functioning, the findings indicate that individuals with dementia who present with the other behavioral disturbances should be assessed for apathy.
The relationship between apathy and cognitive functioning was statistically significant at the bivariate level. However, after Bonferroni’s corrections for multiple comparisons were made and also at the multivariate level, the difference disappeared. Due to a high level of missing data for the cognitive functioning variable, the sample size, and consequently the study power, was greatly reduced for the multivariate models that included the MMSE score. This could explain our failure to observe a statistically significant relationship between apathy and cognitive functioning. It may also be that the MMSE lacks sensitivity in detecting the most commonly found impairments in apathy (i.e., “frontal system” or “executive” impairments).
21The AES-C and AES-I both indicated that levels of functioning (basic and instrumental) were important predictors of apathy. However, due to the cross-sectional nature of the data, we are unable to determine whether higher apathy promotes higher levels of functional impairments, or conversely, whether higher levels of functional impairments lead to increased apathy. An alternate explanation for this relationship is that a third factor, related to the dementia, but not controlled for in the study, might cause both apathy and the functional impairments. Regardless of the direction of the relationship, the results underline the need for the assessment of both level of functioning and apathy in individuals presenting with dementia.
Based on the ascertainment of apathy using the AES-C, the bivariate comparison of apathy in individuals with dementia who lived with their spouse versus those who lived with others (e.g., with children, other relatives, etc.) indicated that those who lived with persons other than their spouse were more likely to score at or above the cutoff value for apathy (40.5). It is important to note that more than half of the individuals with dementia who were living with “others” lived with a family member. Given the fact that apathy in dementia is one of the most problematic of behaviors for family members, it is possible that a higher level of apathy was aversive to caregivers and increased the likelihood of extrusion from the home for those individuals living in other situations. Another possible explanation for this finding is that spouses may have facilitated their loved ones’ participation in a number of daily activities during their daily interactions and as such less apathy was observed in this group by the clinicians. Conversely, other caregivers (e.g., children, other relatives) tend to have competing life priorities such as work or family, which restrict the amount of time available to provide active encouragement to their loved ones with dementia who reside with them. As such, clinicians were more likely to observe apathy in this group than in those with dementia who resided with their spouse. This explanation supports the belief that individuals with dementia and apathy may benefit from therapeutic programs that encourage participation in a range of activities. This hypothesis may be examined using qualitative interviews with spouses and other caregivers of individuals with dementia, with and without apathy, to inquire about the types of activities they engage in on a regular basis and the methods used, if necessary, to engage their loved ones to participate in such activities. The results of such a study would inform clinical practices.
It is important to note that “loss of interest” is a central feature of both apathy and depression and might therefore raise concerns about how to account for the symptom overlap between the two syndromes. Notably, depression might cause apathy, but not all depressed individuals are apathetic and certainly not all apathetic patients are depressed.
21,
38 Furthermore, there is evidence to support the notion that depression and apathy are two distinct syndromes. In our multivariate analyses, the adjustment for the presence of depression in ascertaining the clinical and sociodemographic correlates of apathy partial out this overlapping effect.
Acknowledgments
Dr. Diana Clarke was supported by a Canadian Institute for Health Research Doctoral Award (Grant # 200110MDR-96269-115967), Hy & Bertha Shore and Harry & Sara Gorman Award, and the Toronto Rehabilitation Institute Cognitive Team Excellence Award during the course of this research and the preparation of the manuscript. Drs. van Reekum and Streiner are supported by the Kunin-Lunenfeld Applied Research Unit of Baycrest Centre and are engaged in research supported by the Canadian Institute for Health Research (Grant # 200204MCT-101404-CTAABPI-50298). Dr. Simard is engaged in research supported by a 2005 NARSAD Young Investigator Award. Dr. M. Freedman received grants from the Medical Research Council of Canada and Ontario Mental Health Foundation and received support from the Saul A. Silverman Family Foundation, Toronto, Ontario, as part of a Canada International Scientific Exchange Program (CISEPO) project. The authors thank the research volunteers and the staff and learners in the Departments of Behavioral Neurology and Psychiatry at Baycrest Centre for Geriatric Care.