W ithin the field of psychiatry, eating disorders are classically known for their emergence in adolescent females, notable debility, and their high risk for comorbid axis I and II pathologies. Anorexia nervosa was formally described in the 1870s.
4 Simmonds’
5 report in 1914 linked anorexia with pituitary gland disease, and for at least 20 years, the condition was commonly labeled Simmonds’ disease. Anorexia has the highest mortality rate among psychiatric disorders, with risk of suicide greatest in those with comorbid major depression.
6 –
8 DSM-IV-TR defines three eating disorders: anorexia, bulimia nervosa, and eating disorder not otherwise specified.
9 Anorexia is defined as the refusal to maintain 85% of normal body weight, considerable fear of gaining weight, distorted view of body image or weight, and amenorrhea in females. There are two subtypes of anorexia: restricting type, in which individuals restrict food intake or diet and exercise to lose weight; and binge-eating/purging type, in which individuals may overeat at times and then use methods to purge the food (e.g., vomiting, laxatives, diuretics). Bulimia is defined as recurrent frequent binge eating with recurrent compensatory purging behaviors (e.g., self-induced vomiting, use of laxatives, enemas, diuretics, etc.) and a distorted self-image overly influenced by body shape and weight, but with a normal body weight. Both anorexia and bulimia are more common in industrialized nations and generally begin in late adolescence or early adulthood. Still under investigation is binge eating disorder, currently classified under eating disorder not otherwise specified. The research criteria for this new diagnosis include recurrent episodes of binge eating in discrete periods of time with a lack of control over eating behaviors, intake of more food than necessary, eating when not hungry, and not using compensatory mechanisms. A more in-depth description of the clinical features of these eating disorders can be found in a recent review.
9 As noted in a review of the epidemiology of the major eating disorders, prevalence rates are the most useful for estimating the likely need for care.
7 A recent population-based study analyzed data from the National Comorbidity Survey Replication.
10 The study contains interview-based data that are nationally representative for the United States. Lifetime prevalence in females was estimated at 0.9% for anorexia nervosa, 1.5% for bulimia nervosa, 3.5% for binge eating disorder, and 0.6% for subthreshold binge eating disorder. Lifetime prevalence in males was estimated at 0.3% for anorexia, 0.5% for bulimia, 2.0% for binge eating disorder, and 1.9% for subthreshold binge eating disorder. Overall, these results are similar to previous population-based surveys in the United States and other countries for females.
10 Somewhat higher lifetime prevalence rates for anorexia (1.2%–2.2%) have been reported in female twin studies.
7 As expected, many more females than males fulfilled DSM-IV-TR diagnostic criteria for these disorders. Interestingly, the rate of subthreshold binge eating disorder was much higher in males than in females, resulting in a similar rate when all disorders were combined. Consistent with previous studies, comorbid mental disorders (e.g., mood, anxiety, impulse-control, and substance abuse disorders) were common. Less than half of those interviewed who met the diagnostic criteria for an eating disorder sought treatment for their eating disorder, although more than half reported receiving mental health treatment at some point in their lives. Unwillingness to seek help results in the underestimation of eating disorders in studies based on medical records.
7Multiple studies have reported higher levels of disordered eating in military personnel than in civilian populations.
11 –
16 An early set of studies assessing eating disorder behaviors in Navy personnel found that approximately 50% met criteria for an eating disorder.
11,
12 A later study from the same author that included active duty servicewomen from the Army, Navy, Air Force, and Marines found even higher prevalence rates.
13 All three of these studies were based on anonymous surveys. A prospective study of female active duty Army enlisted and officer personnel used the Eating Disorder Inventory, a self-report questionnaire, as a screening instrument.
14 A full clinical interview by a board-certified psychiatrist was administered to all subjects who met the criteria for being at risk for abnormal eating behaviors (33.6% of the group). Overall, 23% of at-risk subjects (8% of the whole group) were diagnosed as having an eating disorder. A recent study used the Eating Attitudes Survey-26, a standardized self-report survey, to assess disordered eating in entry-level soldiers.
16 The Eating Attitudes Survey-26 has been validated with the DSM-IV eating disorders criteria.
17 The study found that 7% of males and 29.6% of females met criteria for disordered eating.
16 Several studies implicated a concern about passing required fitness assessments as a major factor contributing to the development of disordered eating behaviors in military personnel.
15 Another study used the Eating Attitudes Survey-26 to assess disordered eating in military dependent adolescent girls and their parents.
18 Overall, 8% of adolescents and 9% of parents (10% of mothers, 5% of fathers) met the criteria for disordered eating. Twenty-one percent of adolescents and 26% of parents (27% of mothers, 15% of fathers) met criteria for being at risk for abnormal eating behaviors. This rate was the same for adolescents regardless of the status (active duty or retired) of the military parent, suggesting that risk of disordered eating may remain high even after separation from the military.
Only a few studies have examined eating disorders in U.S. veteran populations.
19 –
21 One set of studies examined the discharge diagnoses of veterans hospitalized in Veterans Affairs (VA) medical centers in 1996.
19,
20 The prevalence of eating disorders was 0.3% in females and 0.02% in males. As noted by the authors, this is likely an underestimation of the actual prevalence, as these rates indicate cases receiving a formal diagnosis. Almost all veterans with eating disorders (95% of women, 92% of men) had at least one comorbid psychiatric diagnosis, compared with less than half of the veterans without eating disorders matched for age, sex, and race. A study of veterans enrolled in a VA women’s health clinic in 1999 found that 9% screened positive for an eating disorder and 45% screened positive for at least one mental health disorder.
21A current review provides an in-depth discussion of the present understanding of the factors that may contribute to the development of eating disorders.
4 As the authors note, the widespread practice of drawing research subjects from patient populations has the potential to bias samples in ways that may distort estimates of risk. The common view that most patients with eating disorders are affluent young white females, for example, may be biased by who has sought treatment. The cultural factor that has been most implicated is the increasingly thin ideal of female beauty, with the internalization of this ideal leading to body dissatisfaction. However, some cross-cultural studies indicate that anorexia can occur in individuals without a fear of fatness. The authors emphasize the importance of studying biological and cultural risk factors together as well as the need for studies that include racial and ethnic diversity.
4Two new studies may shed light on the biological substrate for the differential vulnerability of females and males in the development of eating disorders.
22,
23 Both studies assessed the risk of developing disordered eating in same-sex and opposite-sex twin pairs. One study reported that the prevalence of anorexia (broad diagnostic criteria) is highest in female-female twin pairs (0.99 monozygotic, 1.12 dizygotic), intermediate in female-male twin pairs (0.71 female, 0.60 male), and lowest in male-male twin pairs (0.07 monozygotic, 0.02 dizygotic).
22 The other study evaluated disordered eating as measured by total scores on the Minnesota Eating Behavior Survey in twin pairs.
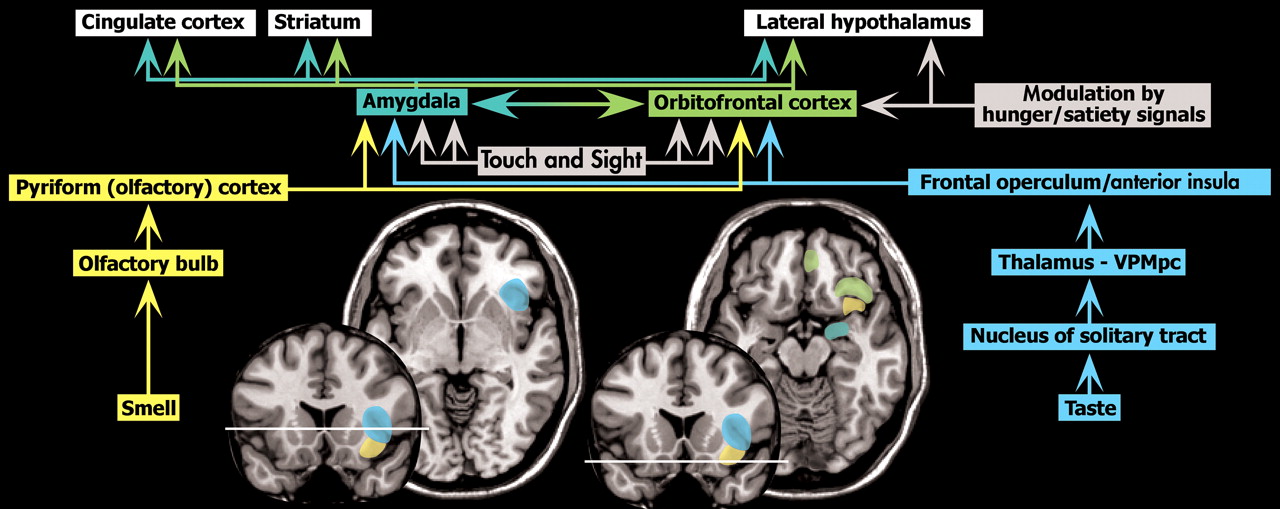
23 Having a male co-twin was associated with lower scores (fewer symptoms of disordered eating) for both male and female twins. Thus female-female twin pairs had the highest scores, followed by opposite-sex females, then opposite-sex males, with male-male twin pairs scoring lowest. Both studies reviewed the literature supporting the importance of in utero influences on the development of many characteristics, including eating behaviors. Sexually dimorphic brain regions that have been implicated in regulation of food intake and body weight include the anterior cingulate, the dorsolateral, prefrontal, and orbitofrontal cortices, the amygdala, the hypothalamus, and the insula.
23 These regions include areas that are directly involved in the sensations of taste and smell, reward value of food, and control of food intake (
Figure 1 ).
1,
2,
24 –
26Anorexia nervosa (primarily) and bulimia nervosa have been the subject of traditional imaging studies for over 20 years. A variety of neuroanatomical structures have been identified as abnormal in either the acutely ill or recovered state. Results have varied with study design, imaging type, individual markers of the illness studied (e.g., interoceptive awareness), and stage of illness. Key learning points from these studies include the range and complexity of circuit abnormalities that may produce the same outward symptoms, the breadth of imaging techniques available for study use, and the potential that identifying the areas of abnormality within a disease group might eventually lead to more refined treatments. Complex psychiatric illnesses are influenced by multiple biological, psychological, and social factors that create the final symptom picture. Within the eating disorders group, many of the biological and psychological factors have been studied with imaging. A critical challenge will be how to integrate sustained individual imaging findings into a global view of the disease process or an anatomical explanation for the symptom complex. To date, there is not a single type of imaging study that can fully demonstrate the entire biopsychosocial picture that creates anorexia or bulimia. Thus, the clinician and scientist gain small pieces of the picture with each imaging type and gradually assemble a fuller perspective. Discussion of the current biological, psychological, and social correlates of eating disorders, with particular emphasis on adolescent development and contributions of the known emotion and behavior circuits of the brain, can be found in several reviews.
9,
27A review summarizing the case report literature related to symptoms of weight/eating changes secondary to brain injury provides some interesting points.
28 Fifty-four cases with documented structural brain lesions associated with symptom presentation were identified. The most commonly reported lesion locations were the hypothalamic area (43%), the cortical area (24%), and the brainstem (13%). The authors noted that the cortical lesion cases more closely resembled classic DSM-IV eating disorders of anorexia or bulimia. Right frontal and right temporal lesions were most commonly noted. The hypothalamic cases had more atypical symptom clusters. The strong comorbidity and familial occurrences with obsessive-compulsive disorder (OCD) was also noted.
A review of the most commonly observed findings in older structural and functional imaging studies noted several weaknesses, including a limited total number of subjects (particularly for bulimia nervosa) and nonspecificity of structural findings across studies.
29 These studies do support nonspecific gray matter loss and probably white matter volume loss in anorexia nervosa. This volume loss partially recovers with increasing body weight. Single photon emission computed tomography (SPECT) and to a lesser extent positron emission tomography (PET) assessments of blood flow/metabolism have been performed in even fewer subjects with varied results. In addition to a mild global reduction, the most common areas of specifically decreased perfusion/metabolism in acutely ill anorexia subjects were the temporal, parietal, cingulate cortices; some areas sustained the abnormalities after recovery. Functional MRI (fMRI) has been used to assess brain activation changes with body image and food challenges. Areas more commonly activated included the prefrontal, anterior cingulate, and parietal cortices—areas heavily tied to the emotion/anxiety circuits. Only a few studies have been completed in subjects with bulimia. Preliminary findings have included diffuse volume reductions acutely on structural imaging and similar decreases on SPECT/PET to anorexia, but with less documentation of resolution in recovery. Brief discussion is also given to potential cortisol interactions, effects of emaciation and dehydration, and the very early work in decreased serotonin binding in these conditions.
More recent structural imaging studies using voxel-based morphometry to assess changes as a function of recovery have not resolved the controversies. One study found a global decrease in gray matter of 1.3% in recovered anorexia patients (median recovery 15.5 months) compared to healthy women.
30 This decrease did not correlate with any clinical measures (e.g., duration of anorexia, length of recovery). Regional analysis indicated widespread cortical and subcortical areas of decreased gray matter. In addition, the anterior cingulate cortex was decreased by 5.4%, correlating with lowest lifetime body mass index (BMI) (i.e., severity of illness). In contrast, normalization of both global and regional brain tissue volumes with recovery (minimum recovery >1 year) were reported for subjects with restricting-type anorexia, bulimic-type anorexia, and bulimia.
31 Decreased volume of gray matter and CSF (but not white matter) relative to comparison subjects and normalization of global measures with recovery (≈6 months) in adolescents with anorexia have also been reported.
32 The recovery-associated increase in gray matter volume correlated with decreased blood cortisol level but not with increased BMI. As the authors noted, the regional differences in gray matter volume that were still present might well be due to the relatively short recovery time.
Functional imaging studies have aimed to refine the areas of altered blood flow before and after treatment (“recovery”) and to identify circuit abnormalities that coincide with commonly found markers of psychological distress in eating disorder patients. Markers include interoceptive awareness, a drive for thinness, bulimia, body dissatisfaction, ineffectiveness, perfectionism, interpersonal distrust, and fear.
33,
34 Three studies have used statistical parametric mapping analysis of regional cerebral blood flow (rCBF) (SPECT) in acutely ill (hospitalized) patients. In one study, subjects with anorexia (N=12, mean BMI=12.5) had decreased rCBF in multiple regions, including the anterior and posterior cingulate cortices, relative to comparison subjects.
35 After treatment (mean BMI=15.6), only the anterior cingulate cortex was decreased relative to comparison subjects. A positive correlation was found between BMI and rCBF in the occipital cortex. In the second study, a positive correlation was found between rCBF in the dorsolateral prefrontal cortex and interoceptive awareness prior to treatment in subjects with anorexia (both restricting and bulimic types, N=8, mean BMI=12.9).
33 Following treatment (mean BMI=18.8), rCBF was significantly increased in the dorsolateral prefrontal, medial parietal, and posterior cingulate cortices and significantly decreased in the putamen. Improved rCBF was also seen in the anterior cingulate and the medial prefrontal cortices, but did not reach statistical significance. No correlations were found between any clinical variables and rCBF following treatment. Of note is the lack of comparison groups and the early scanning for the “after treatment” group. The authors described the subjects as in “partial recovery.” The third study examined patients with restricting-type anorexia (N=31, mean BMI=14.4), binging/purging-type anorexia (N=16, mean BMI=15.3), and bulimia (N=20, mean BMI=20.1).
34 There was a significant positive correlation between the psychological correlates of body dissatisfaction and rCBF in the medial ventral prefrontal and posterior cingulate cortices. Ineffectiveness was positively correlated with rCBF in the lateral ventral prefrontal and posterior cingulate cortices. No correlations were found between rCBF and BMI or duration of illness. This study also lacked a comparison group, did not separate out the subgroups with the imaging results, and did not have any recovered subjects. The authors of these studies attributed the results to known areas of the emotion and behavior circuits that control cognitive and emotional processing. However, it is unclear whether the imaging findings were related to recovery from malnutrition, were related to the disease process itself, or were the final new steady-state for recovery.
One recent study assessed rCBF ([
15 -O] water PET) following a minimum of 6 months recovery, as defined by maintaining >90% average body weight, having regular menstrual cycles, and having no disordered eating patterns.
36 Patients were grouped by diagnosis: restricting-type anorexia nervosa (N=10, mean BMI=20); bulimic-type anorexia (N=8, mean BMI=21); and bulimia nervosa (N=9, mean BMI=23). No areas of abnormal rCBF were found relative to comparison subjects. No relationship was found between rCBF and current BMI. Past lowest BMI was positively correlated with rCBF in the lateral and medial orbitofrontal, medial and lateral temporal, parietal, and sensorimotor cortices for restricting-type anorexia only. The authors of the study noted that recovery of rCBF may be slower than recovery of normal weight. Thus, the stage of recovery should be taken into account in studies using methods based on blood flow, such as fMRI and PET imaging of receptors.
Functional MRI is a useful technique for examining the areas of brain activation under a given task. For eating disorders, fMRI allows a closer look at emotional and cognitive processing in both acute and recovered patients. Tasks have included reward processing, response anticipation and response conflict processing, differentiating positive and negative feedback, and presentation of visual representations of food. Recent studies have demonstrated variations from control subjects in the frontal cortex, cingulate, and caudate under the testing conditions.
37 –
40 These findings may help to differentiate state versus trait characteristics in the future, but generalizing to the disease group as a whole is still premature.
Serotonin (5-hydroxytryptamine, 5-HT) is intimately involved in appetite as well as in the comorbid conditions of anorexia nervosa and bulimia nervosa, such as anxiety, OCD, and depression.
27 It has been suggested that abnormalities in the serotonin system might be present in eating disorders. Early studies indicated that patients who had recovered from anorexia or bulimia had serotonin abnormalities including elevated levels of the metabolite 5-hydroxyindoleacetic acid in their CSF (indicating increased serotonin release).
27 Recent PET and SPECT studies have assessed the state of the serotonin system by imaging binding to several receptors.
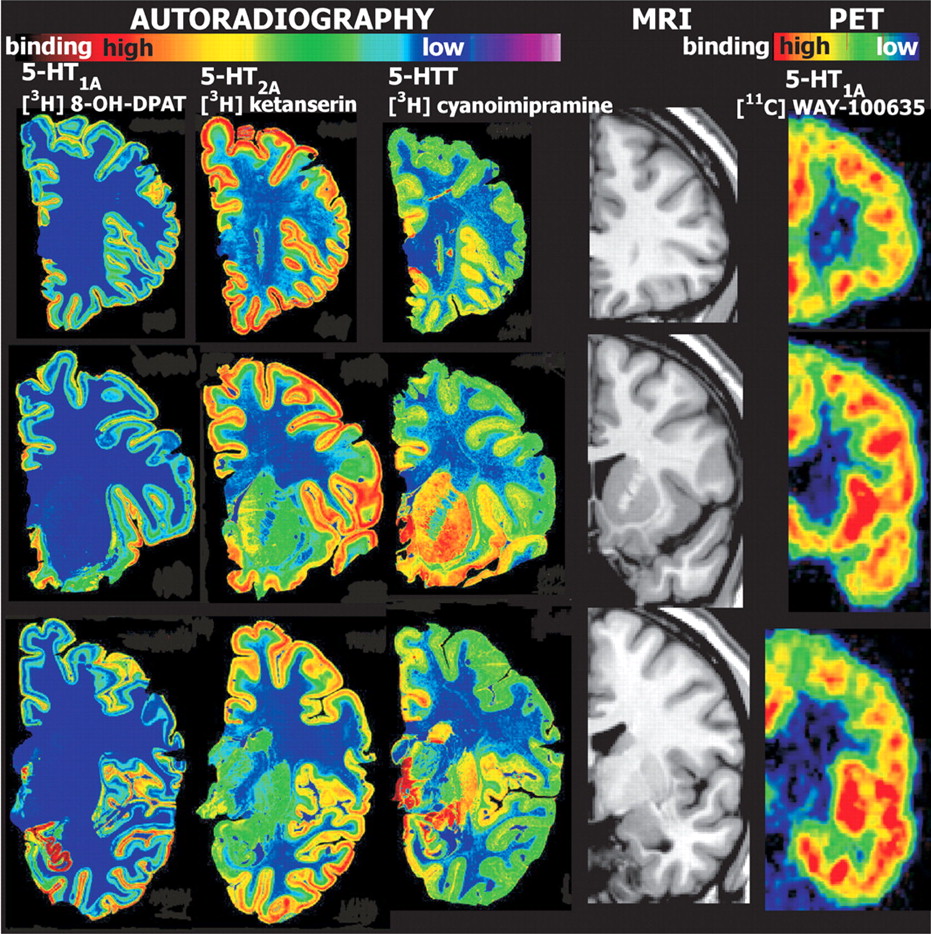
27The 5-HT
1A receptor has two different localizations and functions (
Figure 2 ). In the raphe nucleus it is located presynaptically and serves as an autoreceptor, decreasing serotonin neurotransmission. In other areas (e.g., frontal cortex, hippocampus) it is located postsynaptically, mediating serotonin neurotransmission. Several studies have evaluated 5-HT
1A receptor binding in subjects with eating disorders.
41 –
44 A study of acutely ill subjects with bulimia found that binding potential was increased (7% to 27%) relative to comparison subjects in all cortical areas studied (frontal and temporal cortex).
41 Statistically significant (without Bonferroni’s correction for multiple comparisons) increases were found in the angular gyrus, medial prefrontal cortex, and posterior cingulate cortex. The authors suggested that such widespread areas of increase may be related more to impaired impulse control than specifically to bulimia. An unpublished study of recovered bulimia subjects relative to a comparison group showed increased binding of 5-HT
1A in multiple areas of the cortex, suggesting that this might be a trait marker for bulimia.
42 A study of acutely ill subjects with anorexia (N=15, eight restricting-type anorexia, seven bulimic-type anorexia) also reported increased binding potential (33% to 71%) in all regions examined for both anorexia groups relative to comparison subjects.
43 After adjustment for multiple comparisons (method of false discovery rate), significant increases were present in the sub- and pregenual cingulate, lateral and medial orbitofrontal, prefrontal, lateral and mesial temporal, and parietal cortices, as well as in the brainstem raphe. In contrast, differences have been reported between subjects with restricting and bulimic-type anorexia following recovery (minimum 1 year).
44 Subjects recovered from bulimic-type anorexia still had increased binding in all areas assessed relative to comparison subjects, whereas subjects recovered from restricting-type anorexia were not significantly different from comparison subjects. There were no differences in binding related to comorbid histories of OCD, depression, or substance abuse. There was a positive relationship between amount of binding in the mesial temporal cortex and harm avoidance scores in the subjects recovered from restricting type anorexia. The authors of the study noted that these findings indicate that abnormal 5-HT
1A binding continues even after recovery and may be associated more with the symptoms of binging-purging than with the overall disease.
5-HT
2A receptors are found postsynaptically and mediate serotonin neurotransmission. This receptor is found in multiple areas of the brain, particularly the cerebral cortex (
Figure 2 ). Several studies have evaluated 5-HT
2A binding in patients with eating disorders, as recently reviewed.
43,
45 –
49 Although an earlier study reported reduced binding in the frontal, parietal, and occipital cortices in acutely ill patients with anorexia relative to comparison subjects, two later studies found no differences between acutely ill subjects with either anorexia nervosa or bulimia nervosa and comparison subjects.
43,
45,
46 None of the studies found a significant correlation between regional binding and clinical variables such as BMI. One of the studies reported positive correlations between binding in the lateral and medial orbitofrontal, supragenual cingulate and parietal cortices, and harm avoidance in anorexia.
43 A fourth study compared binding in acutely ill patients with restricting-type anorexia and bulimia-type anorexia.
47 The only significant difference was reduced binding in the parietal cortex in bulimia-type anorexia compared with restricting-type anorexia. Binding in this region correlated positively with reward dependence (but not other clinical variables) in both groups (lower score on reward dependence was associated with lower binding). A study of subjects recovered from bulimia relative to comparison subjects found an absence of the normal negative correlation between age and binding as well as significantly reduced binding in the medial orbitofrontal cortex, but no significant correlations between regional binding and clinical variables.
48 The authors suggested that this regional decrease in receptor binding may be a result of down-regulation in response to recovery-related elevation in serotonergic neurotransmission. A study of subjects recovered from anorexia relative to comparison subjects found the normal negative correlation between age and binding.
49 In addition, binding was reduced significantly in the pre- and postgenual cingulate and mesial temporal cortices by region-of-interest analysis, and in mesial temporal and middle temporal cortices and subcortical parietal lobe by statistical parametric mapping. The authors noted that the consistent changes in the mesial temporal area, which includes the hippocampus and amygdala, supports results of other studies implicating this region in psychopathology. Based on a comparison of these results in anorexia to their previous study in bulimia, the authors also suggested that it might be possible to identify subgroups based on different regional patterns of changes in binding of 5-HT
1A and 5-HT
2A receptors.
48,
49 A follow-up study reported that patients recovered from bulimic-type anorexia had significantly reduced binding (prior to correction for multiple comparisons) relative to comparison subjects in the subgenual cingulate and parietal cortices, and did not show the normal negative correlation between age and binding.
50 Binding in several regions correlated positively with harm avoidance and negatively with novelty seeking.
The 5-HT transporter (5-HTT) is located presynaptically and mediates reuptake of serotonin, terminating its postsynaptic action. The highest concentrations of this receptor are in the striatum and hippocampus (
Figure 2 ). A SPECT ([
123 I]-2beta-carbomethoxy-3beta-(4-iodophenyl)tropane) study analyzing acutely ill patients with bulimia relative to comparison subjects reported significantly reduced binding (17%) in the thalamus/hypothalamus region.
51 There was a negative correlation between duration of illness and binding (lower binding with longer duration of illness). A PET ([
11 C]McN5652) study evaluating 5-HTT binding following recovery compared female patients recovered from bulimic-type anorexia (N=7), restricting-type anorexia (N=11), bulimia (N=9), and 10 healthy comparison subjects.
52 The PET images were coregistered with MRI. Genotyping for two specific alleles in the promotor region of the 5-HTT gene was also done. The group that had recovered from restricting-type anorexia had significantly increased binding in the antero-ventral striatum and dorsal raphe compared with to the group recovered from bulimic-type anorexia. The group recovered from bulimic-type anorexia also had significantly lower binding in the antero-ventral striatum than the group recovered from bulimia. Binding was not related to clinical variables (e.g., comorbid disorders, length of recovery, current or lowest BMI). No genetic differences were observable between eating disorder subjects and comparison subjects, possibly because of the small number of subjects tested. The authors concluded that these results support the hypothesis that there are indeed differences in serotonin function among the eating disorders.
The majority of studies assessing the serotonin system have been the work of one research group.
27,
42 As noted in their reviews, results have varied with the condition of the subjects (acutely ill or recovered), the subtype of eating disorder, and the receptor ligand used. They have commented that the pattern of results suggests that it might be possible to identify subgroups based on different regional patterns of change in binding of serotonin receptor types (trait markers). They also have noted the future possibilities of receptor imaging on the design of medications and choice of medications within a patient subpopulation. It is important to bear in mind, however, that all of these studies had small numbers of subjects, and replication in larger groups is needed. Additional factors that must be taken into account in future studies are age and gender. Several studies have found gender and age-based differences in binding of 5-HT
1A and 5-HTT.
53 –
55 This may impact not only the expression of eating disorders but the response to currently available serotonin-based medications.
An alternative hypothesis has been elucidated that takes the very different view that most or all of the changes seen in anorexia, including psychiatric symptoms and changes in receptor binding, result from the starvation itself (state rather than trait markers).
56 –
58 From an evolutionary perspective, the physiological responses to severely decreased caloric intake (e.g., increased impulsivity, activity, and aggression) are adaptive during times in which resources are scarce.
59 It has been proposed that physical and mental activation induced by self-starvation, both of which are rewarding, may underlie the genesis of anorexia in vulnerable individuals.
56,
57,
60 A therapeutic approach has been developed based on the possibility that disordered eating is maintained by conditioning.
57,
58,
61 Treatment focuses on teaching a more normal pattern of eating, so that eventually satiety coincides with a normal intake of food. Patients are kept warm following meals to reduce activity. Results from the initial randomized trial (N=16) showed successful treatment outcomes for both anorexia and bulimia, with most (14/16) in remission by a median of 14.4 months (range=4.9–26.5 months).
61 A follow-up study of patients with anorexia assessed treatment response as a function of initial BMI (group 1 <14, group 2 >15.5).
58 Mean time to remission was much longer in group 1 (22 months) than in group 2 (10.9 months). Both groups were followed regularly once remission was achieved, and behaviors remained normal at the 5-year assessment.
Conclusion
The hypothesis that alterations in the serotonin system are important in the pathophysiology of eating disorders is consistent with functional anatomy, but studies have not yet provided a complete understanding of the disease process. In addition, the common finding of no correlation between regional binding and a wide range of clinical variables is challenging. The very limited but promising results reported for a therapeutic approach that focuses on remedial training for disordered eating patterns is quite intriguing. Studies are needed to fully evaluate these two very different hypotheses.