T he hippocampus is thought to play an important role in learning and memory processing, and impairments in memory, attention, and decision-making are among the core abnormalities seen in schizophrenia. It is for this reason that the hippocampus has been one of the most widely studied regions of the brain, and abnormality in hippocampal structure has been one of the most robust findings in schizophrenia. There is considerable evidence from postmortem and in vivo MRI studies that patients with schizophrenia have smaller hippocampi than normal comparison subjects.
1 –
4 Furthermore, volume deficits have been reported in first-episode schizophrenia patients and in some nonpsychotic relatives of schizophrenia probands.
5 –
9 These observations suggest that hippocampal volume reductions may be a trait marker or a biological indicator for genetic predisposition in schizophrenia.
There are reasons to believe that differences exist between anterior and posterior regions of the hippocampus. Animal studies have shown that the primate hippocampus is topographically organized along the longitudinal axis, with the medial parts of the entorhinal cortex projecting to the anterior part of the hippocampus and the lateral parts projecting to the posterior hippocampus.
10 Empirical evidence for an anterior or posterior distinction of the hippocampus has also been observed in functional MRI and proton magnetic resonance spectroscopy imaging.
11 –
12 Moreover, overactivity of the ventral hippocampus has been reported to increase dopamine in the nucleus accumbens, thus making us speculate that the anterior hippocampus may be more relevant to the pathophysiology in schizophrenia.
13 –
14 The anatomic specificity of hippocampal volume change in schizophrenia remains a matter of some debate. While some studies demonstrate selective volumetric deficits within the anterior aspect of the hippocampus,
15 –
16 others have indicated deficits within the posterior hippocampus,
17 –
18 and still others find no evidence for a differential deficit of volume across the anterior-posterior axis.
19 –
20 There are a number of factors that may explain the heterogeneity of these results, including differences in demographic variables, illness characteristics, and methodological inconsistencies in defining the boundaries to divide “anterior” from “posterior.”
In recent years there has been a great impetus world-wide in providing specialized psychiatric services for early detection and treatment of patients with first-episode psychosis. Although these patients present with symptoms of psychosis (delusions, hallucinations, disorganized behavior), they are a diagnostically heterogeneous group of individuals and very often the diagnoses become stable only over longitudinal follow-up. Nevertheless, they provide an exciting opportunity to study psychopathology and neurophysiology of psychotic diseases in the very early stages of the illness and without the confounding effects of psychotropic medications.
The aims of our study were to examine hippocampal volume in minimally treated patients with first-episode schizophrenia spectrum disorders (schizophrenia, schizophreniform, and schizoaffective disorders) relative to healthy comparison subjects and to assess if there were differences in the volume along the anterior-posterior axis.
METHODS
Consecutive referrals to the inpatient and outpatient services of the Early Psychosis Intervention Program (EPIP) in Singapore were invited to participate in the study.
Patients with first episode of schizophrenia spectrum disorders, between the ages of 15 to 45 years, who had received no more than 4 weeks of neuroleptic medication, were recruited. They were excluded if they had a diagnosis of substance abuse, significant medical or neurological illness that could affect brain structure and function, or mental retardation. Comparison subjects matched for age and gender, with no personal or family history of a DSM axis I disorder, were recruited from the general population. All individuals (patients and comparison subjects) were right-handed as determined by the Edinburgh Handedness Inventory.
21 A complete description of the proposed study was given to each participant and written informed consent was obtained. The study was approved by the institutional review board. Patients were assessed using the Structured Clinical Interview for DSM-IV (SCID)
22 for axis I diagnosis. They underwent a second diagnostic assessment 1 year later to confirm the stability of the diagnosis. Where the two diagnoses differed (baseline and 1 year), the diagnosis at 1 year was used. Comparison subjects were screened with the nonpatient version of the SCID.
23 For the patients, duration of untreated psychosis was determined by the time in months from the onset of psychotic symptoms (delusions, hallucinations, disorganized behavior) to the initiation of effective treatment, and the age of onset of illness was also calculated. The severity of psychopathology at first presentation was assessed by the Positive and Negative Syndrome Scale (PANSS)
24 as well the Global Assessment of Functioning (GAF) scale.
25A single session MRI scan was performed on a clinical 1.5 tesla MR system (Signa NVi, GE Medical Systems, Wis.) using a standard quadrature head coil. Patients and healthy comparison subjects were positioned in the magnet in a supine position, with arms relaxed by their side. The entire head volume was scanned in the coronal plane using a high resolution, fast gradient recalled echo (FGRE), 3-dimensional, volumetric, T1-weighted sequence (parameters TR/TE/TI/flip angle: 6.4/1.5/400/20°; field of view: 240×180 mm; matrix: 256×256; slice thickness: 1.5 mm, no interslice gap, number of excitations: 2; scan time: 5 minute 1 sec) for structural-anatomic detail.
Magnetic resonance images were transferred to a SUN Sparc 10 workstation (SUN Microsystems, Mountain View, Calif.) for volumetric analysis using in-house MRreg v.1.6.2 software.
26 Hippocampal volume measurements were performed by an experienced reader blinded to the subject status and left-right anatomic orientation. Images were zoomed to a magnification of factor four with intensity windowing monitored continuously. The volume for each hippocampus was calculated by multiplying the number of voxels within each trace per slice by voxel volume. Currently, there is no standard way of defining the anterior and posterior hippocampus. We decided to use a quantitatively objective method that has been published before.
27 This method is based on the midway bisection of the longitudinal axis of the hippocampus. The anterior end of the hippocampus is marked by the appearance of the amygdala, while the posterior end is marked by the appearance of the converging fornix in the superior direction. Based on the MRI coronal slices, the anterior and posterior portions of the hippocampus were designated by dividing the total number of slices in half; with the larger number of slices assigned to the anterior division when the total slice number was an odd number. The patients and comparison subjects did not differ significantly in the number of MRI slices comprising each hippocampal measurement volume.
We matched comparison subjects and patients by gender and age (<20 years, 20–24 years, 25–29 years, 30–34 years, ≥35 years). Independent sample t tests were performed to compare the height and years of schooling between the patients and comparison subjects, as well as to look at hippocampal volume differences based on gender and between patients and comparison subjects. Group×gender interaction (i.e., male patients versus male comparison subjects and female patients versus female comparison subjects) were also done. A general linear model with grouped-matching as a random factor (to take into clustering effect) was carried out to examine the differences in mean hippocampal volumes of patients versus comparison subjects adjusting for age, height, and years of schooling. Statistical significance was set at p<0.05 (two-tailed).
RESULTS
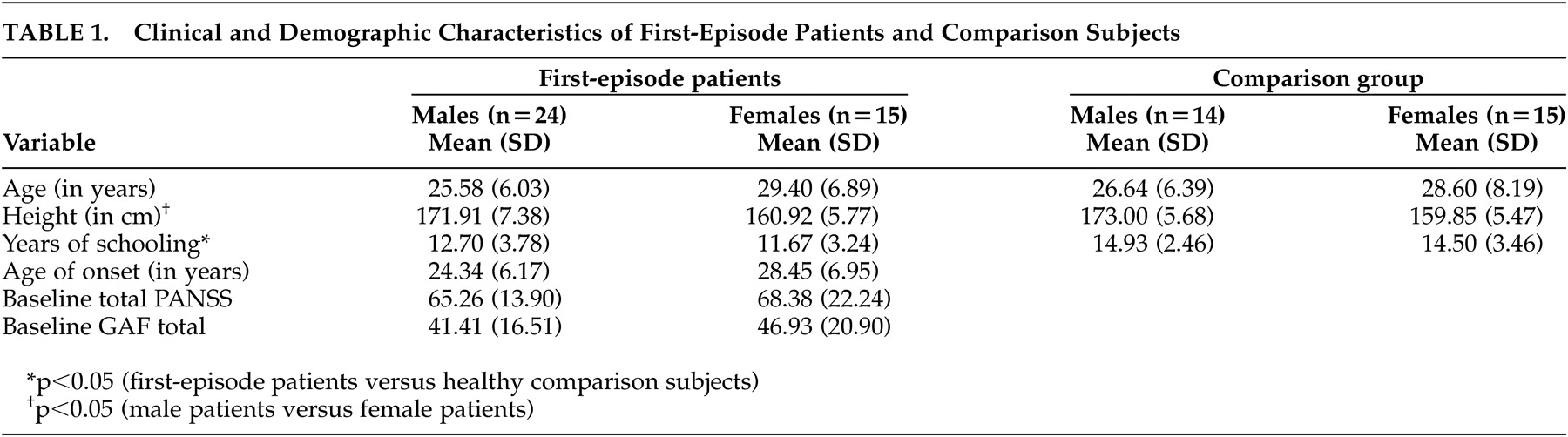
Thirty-nine patients with first-episode schizophrenia spectrum disorders and 29 comparison subjects participated in the study. Among the patient group, 82.1% were Chinese (n=32), 10.3% were Malays (n=4), 5.1% were Indians (n=2), and 2.6% were Eurasians (n=1). Among the comparison group, 69.0% were Chinese (n=20), 13.8% were Malay (n=4), and 17.2% were Indians (n=5). The other demographic and clinical characteristics of the participants are summarized in
Table 1 . The comparison subjects had significantly more years of schooling (p<0.05). Also, the female patients were significantly shorter in height (p<0.05) than the male patients. Female patients did not significantly differ from the male patients in terms of age of onset of illness, or total PANSS and GAF scores.
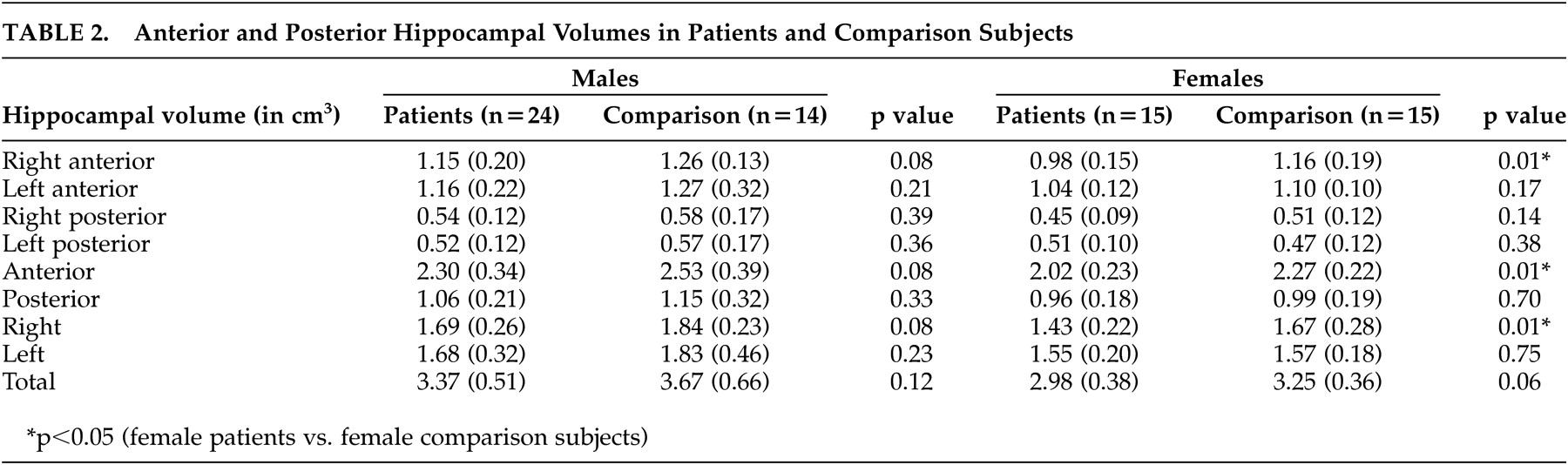
Females had very significantly smaller right, anterior, and total (p<0.005) hippocampal volumes as compared with the males. The right anterior, right posterior, left anterior, right, left, and total hippocampal volumes were also observed to be significantly smaller in the females (p<0.05). The patients had significantly smaller right anterior (p=0.008), right (p=0.015), and anterior (p=0.021) hippocampal volumes relative to comparison subjects.
On analyzing differences between patients and comparison subjects by gender (i.e., splitting the file by gender and comparing volumes between patients and comparison subjects), there were no significant differences in the hippocampal volumes between male patients and comparison subjects. However, the female patients had significantly smaller right anterior (p=0.006), right (p=0.012) and anterior (p=0.006) hippocampal volumes than the female comparison subjects. General linear model controlling for age, gender, height, years of schooling, and diagnosis showed that there was a significant effect of diagnosis and gender on the right anterior (p=0.006 and 0.04, respectively), right (p=0.01, and 0.03), and anterior volumes (p=0.02 and 0.02).
DISCUSSION
Our finding of a smaller anterior hippocampus in patients with first-episode schizophrenia spectrum disorders relative to healthy comparison subjects is similar to that reported in previous studies.
15 –
16 Our patients were scanned as soon as they were stable enough to tolerate the MRI scan, and hence their exposure to antipsychotic medication was minimal. They also did not have a history of substance use. Hence it is very unlikely that the smaller anterior hippocampus was due to the effects of antipsychotics or any other psychoactive substances. Although the patients had significantly fewer years of education than the comparison subjects, there was no significant correlation between the hippocampal volumes and years of education, and statistical adjustment for educational levels did not alter the results. In fact, matching for educational level can be problematic because it would exclude patients with poorer premorbid adjustment caused possibly by prodromal symptoms of psychosis. The anterior hippocampus is predominantly connected with the limbic and striatal systems and, given the hypotheses that failures in the modulation of the limbic and striatal mechanisms are relevant to the pathophysiology of schizophrenia
28 and to the mechanisms of action of antipsychotic drugs,
29 the finding of a structurally smaller anterior hippocampus adds further credence to this theory. The presence of this abnormality at the beginning of the illness may also indicate that a smaller anterior hippocampus may be a trait marker of the illness either due to a genetic susceptibility or early environmental damage, as suggested by studies that have found smaller hippocampal volumes in nonaffected first-degree relatives of patients with schizophrenia as well as linked this finding with fetal hypoxia.
30 In fact, the neonatal ventral hippocampal lesion in the rat has been used as a model of schizophrenia. The same authors have also shown that this early insult to the ventral hippocampus causes reduction in dopamine transporter (DAT) mRNA, although it is unclear whether this reduction coincides with the emergence of behavioral changes in young adulthood or whether it is present from early in life.
31 –
32We also found that there was a gender by diagnosis effect with the female patients showing significantly smaller anterior and right hippocampal volumes than female comparison subjects; an effect that was not seen in the male patients. Male and female patients with schizophrenia do differ in their clinical manifestations with females presenting with later age of onset, better premorbid social adjustment, and better long-term outcomes.
33 –
35 The male and female patients in our study did not differ significantly with regards to their age of onset and severity of psychopathology at the time of presentation, as measured by duration of untreated psychosis, PANSS, and GAF. The mean age of onset in our female patients was later than the male patients but it was not statistically significant. In fact, this is in line with the older age of onset in females. Interestingly the male patients in our sample seemed to have a later age of onset than what is normally seen in schizophrenia, and it is possible that this later onset of illness helped the male patients escape the early insult to the hippocampus.
Some previous studies looking at gender differences in schizophrenia have reported that male patients appear to manifest greater severity of structural impairment and had smaller hippocampal volumes than the female patients.
9,
36 There have been other studies that have not found any significant effect of gender.
3,
37 Our finding of a smaller right hippocampus in females is similar to that reported by Collinson and colleagues.
38 They assessed the volumes of right and left cerebral hemispheres in early onset schizophrenia and found that the female patients had a significantly reduced rightward asymmetry relative to the female control subjects, indicating that in females there is a volume loss that is relatively greater in the right hemisphere.
We note some possible limitations with our study. As a surrogate measure for intracranial volume, height was used. We also did not look at neurocognitive correlates of anterior versus posterior hippocampus that could provide further evidence for anterior and posterior differentiation of hippocampus in patients with schizophrenia spectrum disorders. Lastly, although we found a gender by diagnosis interaction, our study was not primarily designed to look at gender differences; hence this finding needs to be replicated in another study that is powered to look at the effects of gender in patients as well as healthy comparison subjects. Further research exploring anterior-posterior differentiation of the hippocampus using not only neurocognitive measures but also the connectivity with techniques such as diffusion tensor imaging could shed more light into some of these issues.