P arkinson’s disease is a common neurodegenerative disease causing primarily a motor disorder that leads to disability and impairs quality of life.
1 Failure of the effects of pharmacotherapy led to the development of advanced surgical options of which deep brain stimulation of the subthalamic nucleus (STN-DBS) has become the most popular.
2 –
3 STN-DBS has been shown to be effective in reducing both the motor disability and the dose of dopamine replacement therapy along with its related complications.
3 –
4 Deep brain stimulation is an adjustable and reversible technique, and was shown to have minimal or no effect on cognition and behavior.
5 –
6 However, accumulating reports
7 –
9 suggest that behavioral and psychiatric adverse effects have been probably underestimated and underreported. Recent reviews
6,
10 –
11 reported a prevalence of behavioral symptoms that is extremely variable, ranging between 0.5% and 75% within newly operated patients as well as during long term follow-up. While different medical centers performing deep brain stimulation reported increased apathy,
12 –
13 emotional reactivity,
14 hypomania,
15 depression,
8 –
9,
14 and suicide
8 –
9,
16 following implantation, other studies showed minimal behavioral complications, mostly transient and treatable in nature.
17 –
19 Possible explanations for these complications may be the mislocation of the stimulating electrodes causing a “stimulation-locked depressive state,”
20 suboptimal stimulation parameters
21 or rapid dopamine replacement therapy reduction or withdrawal.
22 –
23 In addition, psychological variables may themselves play a role in the onset or the exacerbation of behavioral symptoms, such as social adjustment difficulties
24 and premorbid psychiatric vulnerabilities.
14 While these adverse effects may considerably compromise the overall outcome of STN-DBS in patients with Parkinson’s disease, factors associated with them have not yet been determined
6 and it is not clear which patient profile is at a higher risk for behavioral decline.
25 The aim of the current study was to evaluate the change in the neurobehavioral and the neuropsychiatric symptoms following STN-DBS and to identify possible risk factors for their occurrence.
METHODS
Patients
The study population consisted of 25 patients with early- and late-onset idiopathic Parkinson’s disease according to the U.K. Parkinson’s Disease Society Brain Bank clinical diagnostic criteria.
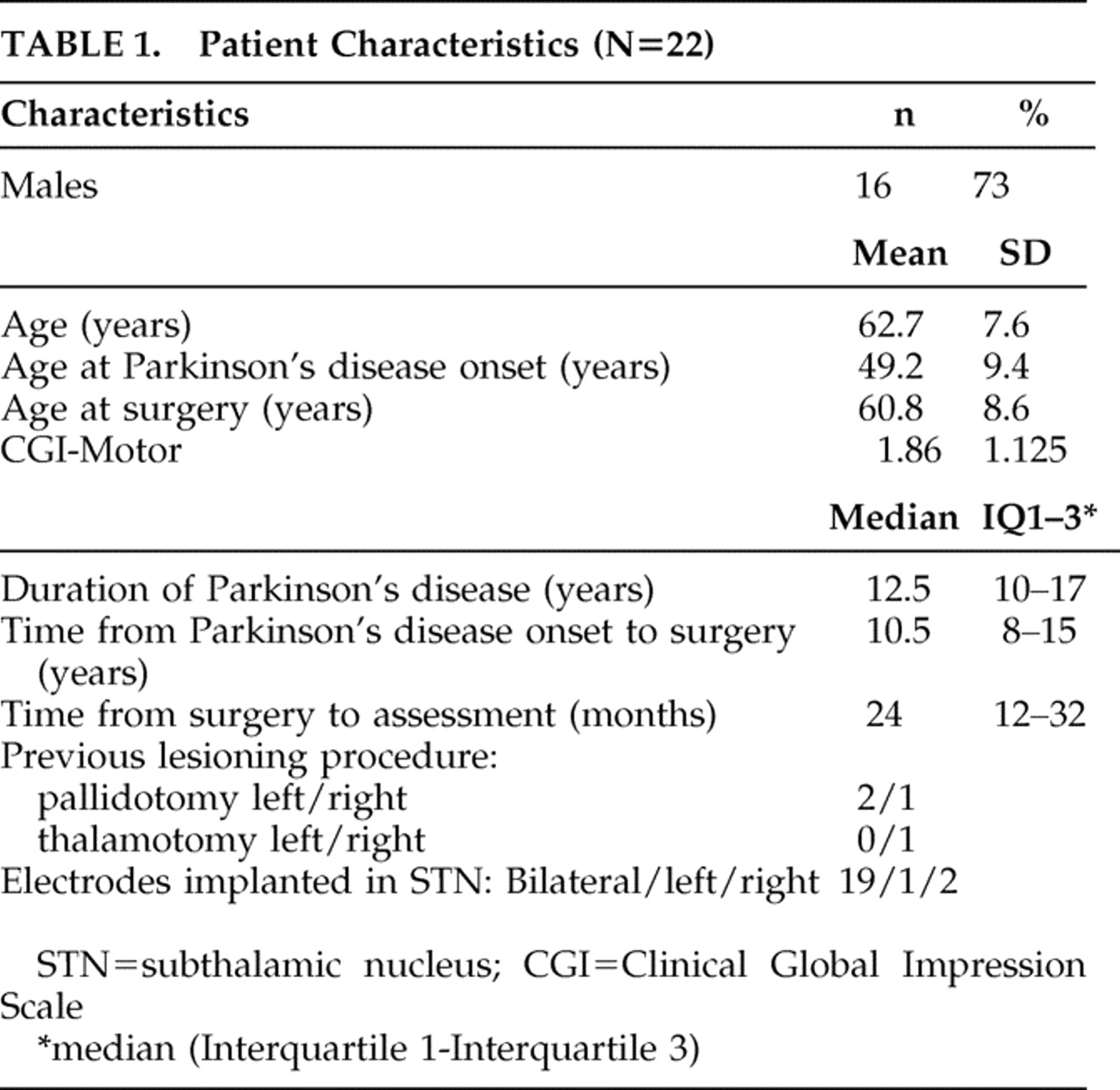
26 Patients were referred for surgery because of severe disability despite optimized dopaminergic therapy, including severe “off” state parkinsonism, motor fluctuations, and dyskinesias. Patients were excluded for surgery, as accepted, if they had other unstable medical or neurological problems, active psychiatric symptoms, or prominent cognitive decline. Most patients underwent surgery in centers outside of Israel (n=13). Implanted patients were then followed up at our center during data collection. Two patients were excluded from the study due to language limitations and one patient due to missing data. The patient characteristics are summarized in
Table 1 . All patients included in the study provided informed consent, and the study was approved by the institutional review board.
Patient Evaluation
The medical charts of the remaining 22 patients were reviewed for demographic data, clinical aspects of Parkinson’s disease, pharmacotherapy, and stimulation parameters. The patients and their caregivers were interviewed by a neurologist (OSC or SH) or a psychologist (OP) in person and by telephone using clinician and patient rating scales. They were asked to rate several aspects of behavior of the patients during the 3 months before the present assessment and retrospectively for the 3 months preceding the STN-DBS operation.
The patient’s interview included the following tests:
The Neurobehavioral Rating Scale
The 27-item Neurobehavioral Rating Scale
27 is an observer-rated instrument for a broad range of cognitive, psychiatric, and behavioral disturbances in patients with various neurological conditions which measures seven domains, namely: cognitive impairment, agitation, retardation, depression, apathy, psychosis and lability. The Neurobehavioral Rating Scale showed high interrater reliability (Spearman’s correlation coefficient =0.93, p<0.001)
28 and satisfactory convergent validity with other cognitive and behavioral measures in patients with Parkinson’s disease in its modified version.
29The Brief Psychiatric Rating Scale
The Brief Psychiatric Rating Scale
30 was originally designed to measure behavioral changes in patients with schizophrenia but has been widely used as a rating scale in clinical trials for antipsychotic agents in patients with Parkinson’s disease. Interrater reliabilities (Pearson coefficients) vary from 0.63 to more than 0.80, and correlations with measures of positive and negative symptoms (concurrent validity) are adequate (0.92 and 0.82, respectively).
31 The Brief Psychiatric Rating Scale has a suicide subscale that was added for the patient assessment (by the clinician) since it was absent in the Neurobehavioral Rating Scale.
The Beck Depression Inventory
The Beck Depression Inventory (BDI)
32 is a commonly used and well validated self-rating scale for depression in Parkinson’s disease.
33 Cronbach alpha for the BDI in Parkinson’s disease is high (0.88), and a cutoff of 14/15 was calculated for patients with Parkinson’s disease showing the highest sensitivity (0.71) and specificity (0.90).
34The caregiver’s interview included the following tests:
The Neuropsychiatric Inventory
The Neuropsychiatric Inventory
35 is designed primarily to assess psychopathology in patients with dementia, assessing severity (of delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, abnormal motor output) and the resulting caregiver’s distress from each of the symptoms measured. The Neuropsychiatric Inventory has shown good internal consistency (Cronbach alpha 0.87–0.88) with an interrater agreement of 90%–100%. Concurrent validity was established with a correlation of 0.66 for frequency and 0.71 for severity of symptoms to other measures of behavior in dementia.
35Assessing the Dopamine Dysregulation Syndrome
Three questions were formulized according to the principles laid down by previous authors.
36 The questions to the caregiver were phrased “During the 3 months that preceded the deep brain stimulation operation, how severe was the need of the patient for (a) sexual activity, (b) exaggerated consumption of food, and (c) compulsive behavior such as gambling, drug hoarding, shopping and spending money?” Scores ranged from 0 (none) to 4 (a permanent and devastating need).
The Work and Social Adjustment Scale
The Work and Social Adjustment Scale
37 is a measure of disability, functional impairment at work, home, leisure and personal relationships. Psychometric properties have been tested in populations suffering from a variety of neuropsychiatric conditions, such as depression, OCD, phobias and additional health conditions.
38,
39 The authors showed internal consistency (Cronbach alpha) of 0.70–0.94, test-retest reliability of 0.73, interrater reliability of 0.86, and concurrent validity with depression scores of 0.76.
37In order to identify risk factors for behavioral worsening following STN-DBS we used a Clinical Global Impression scale.
40 This is a seven-point categorical scale that provides a single global rating of change from baseline (1=marked improvement, 2=moderate improvement, 3=minimal improvement, 4=no change, 5=minimal worsening, 6=moderate worsening, 7=marked worsening). Any change recorded on a Clinical Global Impression scale was considered clinically relevant by definition. Although it has a moderate interrater reliability (0.41–0.66), the scale has been used in several large randomized Parkinson’s disease psychosis clinical trials and appears to be sensitive to change.
31The same investigator throughout the study made the Clinical Global Impression scale assessments for a given patient, and all assessments were done at approximately the same time of day for all visits.
The Clinical Global Impression (CGI) scale was used in the present study to divide the patients into two behavioral outcome groups (CGI-Behavior): those graded 1 (marked improvement) to 4 (no change) were assigned to the “stable/improved” group and those with scores of 5 (minimal worsening) to 7 (marked worsening) to the “worsened” group. The two groups (n=11 each) were then compared for preoperative behavioral status, disease characteristics, stimulation parameters, medication changes after deep brain stimulation and demographic variables. As most patients were operated on outside Israel, uniform and formal rating of their motor status before the operation was missing. In order to assess the motor benefit they obtained from STN-DBS, we used a Clinical Global Impression scale for motor improvement (CGI-Motor) rated by the same neurologist after a thorough interview with the patient and caregiver while examining the medical records.
Statistical Analysis
We used a matched pair, before and after design to evaluate the impact of STN-DBS on the behavioral and psychiatric profile of the patients. Using the Neurobehavioral Rating Scale, the Neuropsychiatric Inventory, the Brief Psychiatric Rating Scale, the BDI, the Work and Social Adjustment Scale scales, and the dopamine dysregulation syndrome scores, we measured changes in behavioral functioning of the total group by comparing their ratings before the operation and during the last 3 months. The McNemar test was computed to assess the difference between the proportions of behavioral and psychiatric complications in patients before and after the deep brain stimulation operation. The Wilcoxon signed-ranks test for paired samples was used to compare the scores of the different rating scales pre- and postoperatively, and the Spearman correlations were used for nonparametric data, namely the depression rating of the patients and their caregivers before and after STN-DBS.
To compute effect size estimates for the nonparametric tests conducted, the raw data were transformed into rank values for which the eta squared statistic (η 2 ) was calculated.
Comparisons of baseline characteristics between the two outcome groups were performed with nonparametric Mann-Whitney test for continuous variables and Fisher’s exact test for categorical variables as well as Phi and Cramer’s V tests. Continuous variables were expressed as mean ± standard deviations or median (25th percentile–75th percentile). Discrete data were given as counts and percentages. Fisher’s exact tests were applied to assess the relation between the preoperative behavioral scores (Neurobehavioral Rating Scale, Neuropsychiatric Inventory, Brief Psychiatric Rating Scale, BDI, the Work and Social Adjustment Scale scales, and the dopamine dysregulation syndrome scores) and the behavioral outcome. Spearman correlation was used to evaluate the relation between the motor (CGI-Motor) and behavioral (CGI-Behavior) outcomes.
RESULTS
Twenty-two patients with Parkinson’s disease treated with STN-DBS were included. The group characteristics are given in
Table 1 . According to the CGI-Motor score, 12 patients (50%) scored 1 (very much improved), six patients scored 2 (much improved), one patient scored 4 (no change), and one scored 5 (minimal worsening). The mean CGI-Motor score was 1.86±1.125.
Total Work and Social Adjustment Scale scores ranged from 21.0±12.2 before the operation to 19.5±14.1 during assessment (out of a maximum of 40 points), indicating that social and work functioning was moderately impaired before and remained the same after STN-DBS (p values of all five subscales of the Work and Social Adjustment Scale were not significant for the McNemar test).
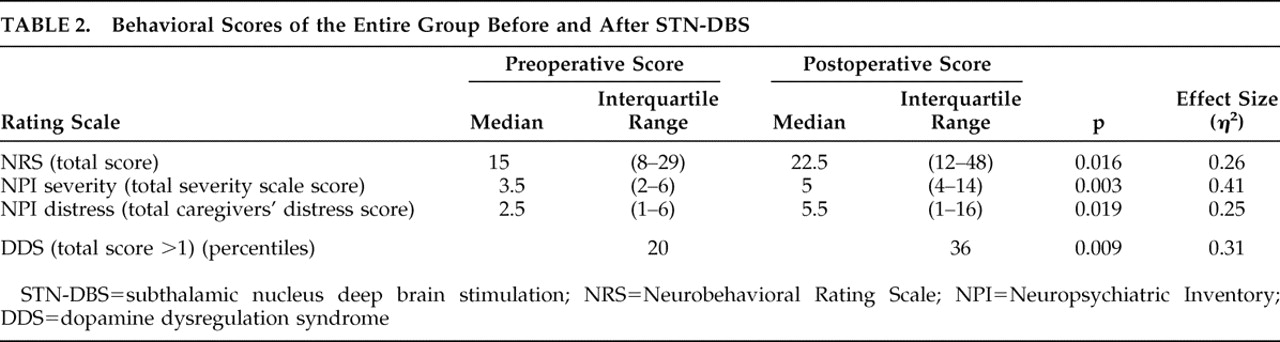
Total group severity measures of behavioral symptoms are compared for before and after the operation in
Table 2 .
Further analysis of the Neuropsychiatric Inventory item scores of the whole group before and after STN-DBS, showed a significant increase of apathy (p=0.020, η 2 =0.25) and anxiety (p=0.039, η 2 =0.19). There was no evidence of an overall increase in depression (p=0.959) using the Wilcoxon signed-ranks test.
No correlation was found between the severity of depressive symptoms before and after the operation when looking at the ratings of preoperative caregiver’s Neuropsychiatric Inventory severity scale and the present BDI score given by the patient (Spearman’s ρ=0.346, not significant).
Based on the caregiver’s impression given by the Neuropsychiatric Inventory, the predominant behavioral disturbances before STN-DBS were symptoms of depression (12 cases), followed by nighttime behaviors (10 cases), irritability or lability (nine cases) and then agitation, anxiety and apathy (seven cases each). A few cases of changes in appetite (four), disinhibition (two), delusions (one), and motor disturbances (one) were also reported. According to the Neuropsychiatric Inventory, the symptoms that appeared anew in highest frequency were apathy and anxiety (five cases) followed by hallucinations and appetite changes (four cases), depression, delusions and nighttime behaviors (three new cases each). The percentage of patients reporting dopamine dysregulation syndrome symptoms (score >1) changed from 20% to 36%, p=0.009 (η 2 =0.31).
Depressive symptoms had resolved in four patients and anxiety in one. Suicidal ideation as measured by the Brief Psychiatric Rating Scale was evident in three patients before the operation and was reported in seven new patients after the operation, a total of nine patients (p=0.046, η 2 =0.18). One of the patients, a 76-year-old man with Parkinson’s disease for 10 years, was apparently suicidal before the operation, but did not share this information with the medical personnel. This patient had performed an unsuccessful suicide attempt 1 month after surgery and was admitted to a psychiatric ward for a short hospitalization. Two months later he accomplished suicide. His motor condition following STN-DBS had much improved (CGI-Motor=2) while his behavioral condition had worsened significantly during postsurgical assessment (CGI-Behavior=6). According to his wife he was severely depressed and agitated before the operation (Neuropsychiatric Inventory depression severity=3, agitation severity=3), but he himself reported only mild depression (BDI score=14).
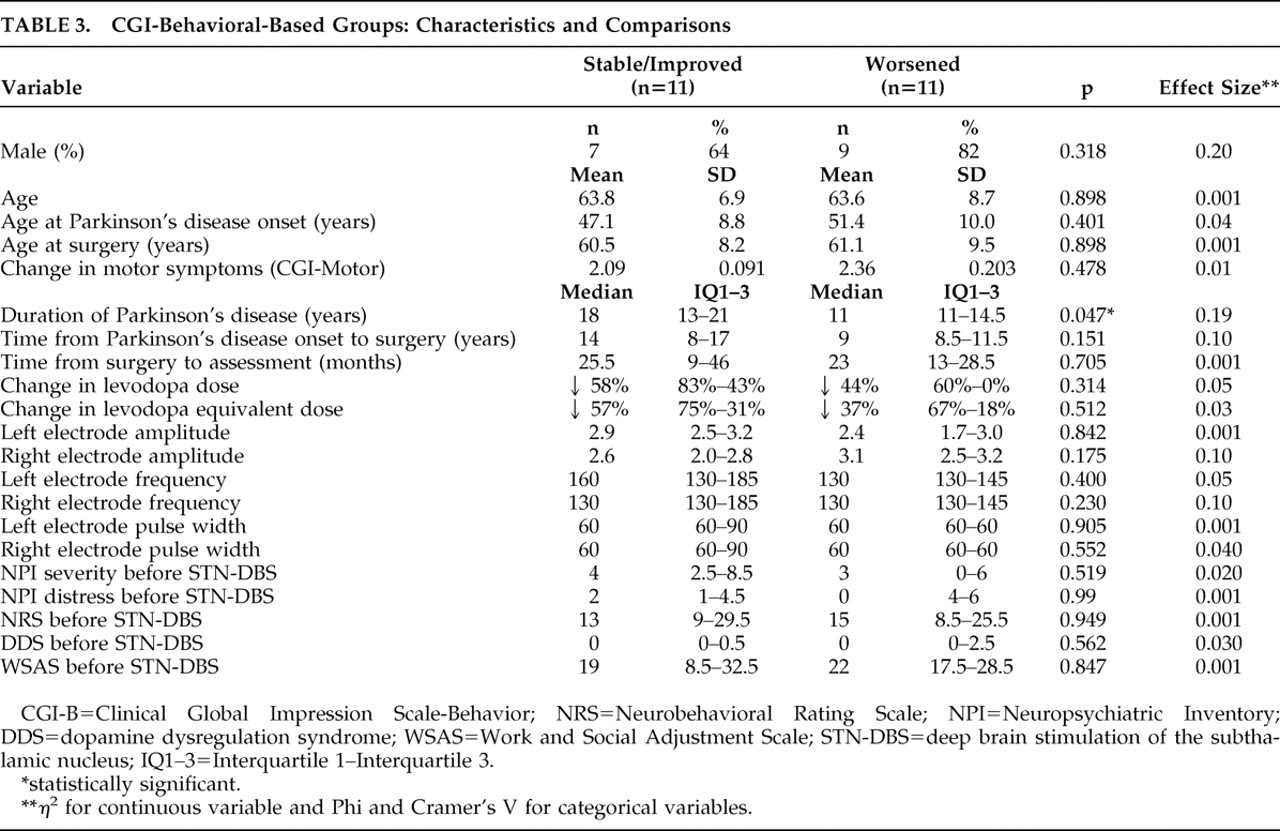
When comparing the two behavioral outcome groups, no significant differences were found in terms of gender, age at assessment, age at disease onset or age at the time of surgery. However, the disease duration was significantly shorter for the patients who had deteriorated behaviorally compared to those who were stable or improved (11 [11–14.5] versus 18 [13–21], p=0.047; η
2 =0.19) (
Table 3 ). Concordantly, there was a trend for a shorter time from the onset of Parkinson’s disease to surgery for the “worsened” group compared with the “stable/improved” group. No differences were found in the average reduction of
L -dopa or
L -dopa equivalent dose following the operation or in stimulation parameters (
Table 3 ). There was also no correlation between the motor (CGI-Motor) and behavioral (CGI-Behavior) outcomes (ρ=0.231, p=0.3). The preoperative behavioral scores (according to the Neuropsychiatric Inventory, the Neurobehavioral Rating Scale, the BDI, the Neuropsychiatric Inventory, the Brief Psychiatric Rating Scale, the Work and Social Adjustment Scale and items addressing the dopamine dysregulation syndrome) were similar for both groups. Patients in the behaviorally deteriorated group were found to have a lower incidence of aggression or agitation (Neuropsychiatric Inventory caregivers’ ratings) prior to operation (45.5% versus 90.9%, p=0.032; Phi & Cramer’s V=0.628) (
Table 3 ).
DISCUSSION
In our group of patients with Parkinson’s disease treated with STN-DBS, several behavioral symptoms worsened in terms of prevalence and severity and previously asymptomatic patients became affected. Behavioral features varied, and were not clustered around depression, anxiety, psychosis, or impulse control disorders (dopamine dysregulation syndrome). Apathy, anxiety, and suicidal ideation increased significantly after the operation, while depressive symptoms appeared stable. The worsening of the behavioral symptoms and the social adjustment of the patients were dissociated from the improvement in motor disability, as pointed out in other studies.
19,
24,
41 Similarly, no correlation was found between the motor and the behavioral outcome (Clinical Global Impression scale).
Previous studies including short and long term follow-up of patients after STN-DBS implantation generally support the safety of this procedure
4,
7 and report much lower rates of acute and long term behavioral adverse events.
10 –
11,
17 –
19 The high incidence of behavioral symptoms in our STN-DBS patients may reflect the sensitivity of the various tools we chose and the information obtained from the caregivers. It should be kept in mind that behavioral symptoms were reported with rating scales and not by standard DSM-IV criteria,
42 thus reflecting patients’ complaints, not diagnosis.
Behavioral deterioration following STN-DBS was not associated with gender, age or other demographic features except for a finding that the patients who worsened behaviorally following STN-DBS had significantly shorter disease duration and were admitted to this treatment earlier in the course of their disease. One possible explanation for this finding is that in those patients the rate of disease progression was more rapid up to a point of severe disability, necessitating earlier surgical intervention. Thus the prominent psychiatric complications may reflect a more aggressive nature of their illness, resulting in a more intense neuronal loss both in the “motor” and the “complex” dopaminergic pathways.
43 This rapid neurodegeneration may prominently contribute to both motor and nonmotor aspects of the disease.
44A disappointing finding is that preoperative behavioral symptoms did not differ between the outcome groups, did not correlate with postoperative scores, and thus cannot serve as predictors or warning signs for these unwanted behavioral outcomes.
According to previous reports, an association may be found between changes in medication after STN-DBS and depression or apathy,
23 but we found similar dose adjustments in both groups. Acute and long-term mood and behavior changes were previously reported to be induced by stimulation.
13,
45 However, we found no differences between the groups in the stimulation parameters. We had no data concerning the exact location of the electrodes within and relative to the subthalamic nucleus, therefore these data could not be analyzed.
This study has several limitations. As a retrospective study, there could be a limited reliability of the behavioral data concerning the time before the operation. Quantitative motor assessments before and after STN-DBS supporting the positive motor outcome are lacking. There is variability in surgical indications, surgical procedures, and time from surgery to assessment within the patient population. As neuropsychiatric and neurobehavioral rating scales were used rather than semistructured psychiatric interviews, formal psychiatric diagnoses are lacking and this limits our ability to generalize these findings.
46 The absence of a non-surgical Parkinson’s disease control group limits our ability to assess the impact of STN-DBS on the natural course of Parkinson’s disease and its nonmotor, neuropsychiatric complications. Lastly, the small sample size of the study may result in low statistical power and Type II errors.
The overall success of STN-DBS in Parkinson’s disease depends on a general patient outcome, including motor, behavioral, and social aspects. It is therefore of great importance to find tools for identifying patients at risk for deterioration.
6,
25 While selection criteria for STN-DBS have been previously defined,
47 predictors of a safe behavioral outcome are still lacking. Risk factors for behavioral deterioration have not yet been determined and, at present, only patients with a notable cognitive decline or active psychiatric symptoms such as depression or psychosis are generally ruled out for surgery until treated and improved.
As has been reported in other studies, one patient in this study accomplished suicide despite having improvement in motoric symptoms. It should be emphasized that in our sample there was a very high rate of postoperative suicidal ideation (9 of 22 or 44% of all patients) and that despite no overall worsening in total depressive symptoms of previously nonsuicidal patients, seven of 19 (37%) developed suicidal ideation postoperatively.
Since motor outcome and preoperative psychiatric symptoms do not correlate with postoperative psychiatric complications and given the high rate of postoperative suicidal ideation with no clear predictive factors, it is essential that all patients be routinely evaluated and closely followed for suicidality. This can be done using a thorough assessment of suicidal symptoms by a psychiatrist before the operation as a part of the selection process. The use of sensitive rating scales as well as structured clinical interviews is also preferable. Patients who develop suicidal symptoms following implantation should be closely monitored by a psychiatrist and treated promptly.
Future studies should aim at understanding the causes for emergence of behavioral symptoms in patients with STN-DBS and finding ways to prevent them.