P arkinson’s disease is a chronic, progressive neurodegenerative disease characterized by bradykinesia, rigidity, tremor, and postural instability, and is second only to Alzheimer’s disease as the most common neurodegenerative disorder.
1 Parkinson’s disease can have substantial impact on patients’ quality of life and places a large burden on patients, caregivers, and the health care system. The total economic burden of Parkinson’s disease in the United States has been estimated at $23 billion annually, of which almost 70% is related to lost productivity and uncompensated care provided by family members.
2 While great strides have been made in the treatment of Parkinson’s disease-related motor symptoms, associated neuropsychiatric symptoms remain a major cause of disability.
3 These psychiatric symptoms have been appreciated as distinct Parkinson’s disease diagnostic clusters.
4 Studies have indicated that psychosis is one of the dominant symptom clusters, and early treatment may be effective in slowing psychosis progression.
5 However, until recently, no standardized diagnostic criteria for associated psychosis in Parkinson’s disease, sometimes referred to as Parkinson’s disease-induced psychosis (PDP), existed.
6 This absence of an accepted definition has complicated epidemiologic studies of PDP, such that the true prevalence of PDP is unknown.
METHODS
Data Source and Study Design
Using the criteria set forth by the NINDS/NIMH working group, we conducted a retrospective analysis of a de-identified health care claims database from a large United States managed care population to estimate the point prevalence of PDP within this population.
The managed care provider consists of primarily discounted fee-for-service independent practice association model health plans. The database is updated frequently and includes enrollment data and electronic pharmacy and medical claims for approximately 15 million commercial health plan members annually, with claims dating back to 1993. The patient population is geographically diverse across the United States, with the greatest proportions of members in the Midwest and South regions. The data were accessed using Health Insurance Portability and Accountability Act (HIPAA)-compliant techniques, and no identifiable protected health information was extracted during the course of this study.
We identified patients with Parkinson’s disease who were enrolled in the health plan as of September 2007 (the date through which complete claims data were available), and we examined up to 10 years of claims history while the patient was continuously enrolled to determine the presence of PDP.
Participants
Patients with a primary or secondary diagnosis of primary/idiopathic Parkinson’s disease (ICD-9-CM: 332.0) were selected, and the date of patients’ first diagnosis of Parkinson’s disease during their period of continuous enrollment prior to (and including) September 2007 was assigned as the index date. Patients were required to have a second diagnosis of Parkinson’s disease at least 30 days after index date, and patients were excluded if they had evidence at any time during the study period of secondary parkinsonism (ICD-9-CM: 332.1); dementia with Lewy bodies (ICD-9-CM: 331.82); or primary psychiatric disorders such as schizophrenia, including schizoaffective disorder (ICD-9-CM: 295.xx), other mood disorders with psychotic features (ICD-9-CM: 293.83, 296.0x-296.90), or delirium (ICD-9-CM: 290.11, 290.3, 290.41, 291.0, 292.81, 293.0–293.1). They were also excluded if they had evidence of delusional disorder (ICD-9-CM: 291.5, 292.11, 297.x) during the preindex period.
Study Measures
Within the Parkinson’s disease population, the prevalence of PDP was estimated using the criteria developed by the NINDS/NIMH working group.
7 In order to define a range of possible prevalence rates, three different claims-based definitions that approximated the NINDS/NIMH criteria were tested. The standard definition required ≥1 medical claim with a diagnosis of psychosis (ICD-9-CM: 298.0, 298.1, 298.4–298.9), hallucinations (ICD-9-CM: 293.82, 368.16, 780.1), or delusions (ICD-9-CM: 293.81, 297.1). The strict definition required ≥1 medical claim with a diagnosis of psychosis and ≥1 medical claim with a diagnosis of hallucinations or delusions. The time-dependent definition, which most closely represents the NINDS/NIMH working group criteria, was applied to the patients qualifying under the standard definition but required a second claim with a diagnosis of psychosis, hallucinations, or delusions at least 30 days after the initial claim.
Patient demographic characteristics and treatments were examined to describe the Parkinson’s disease and PDP populations. Additionally, medical claims during the period of continuous enrollment prior to September 2007 were examined for presence of dystonia (ICD-9-CM: 333.6–333.8x, 781.0) or dementia (ICD-9-CM: 290.xx, 291.2, 292.82, 294.1x, 294.8, 331.0–331.2, 331.83), as well as surgical treatment for Parkinson’s disease, i.e., intracranial neurostimulation (current procedural terminology: 61863–61868, 61875; ICD-9 procedure: 02.93) and pallidotomy or similar via stereotactic surgery (current procedural terminology: 61720, 61735; ICD-9 procedure: 01.42, 92.30, 92.39). Patients’ pharmacy claims were also examined, and use of Parkinson’s disease medications (i.e., L -dopa, dopamine agonists, anticholinergics, monoamine oxidase type B inhibitors, catechol-O-methyltransferase inhibitors, amantadine, rivastigmine) and atypical antipsychotics was determined.
Statistical Methods
To characterize patients with Parkinson’s disease and with PDP, we examined patient characteristics (gender, age) for the identified Parkinson’s disease population and each of the three PDP populations (standard, strict, time-dependent). We contrasted patient characteristics and associated clinical features (presence of dystonia or dementia, Parkinson’s disease treatment, and antipsychotic use) for the largest group of PDP patients (i.e., those meeting the standard criteria for PDP) versus Parkinson’s disease patients who did not meet the standard PDP criteria. Pearson chi-square tests and t tests required p<0.05 for significance. Parkinson’s disease psychosis prevalence estimates are presented per 1,000 patients with Parkinson’s disease, with exact 95% confidence intervals based on the binomial distribution. Data extraction and statistical analyses were conducted using SAS 9.1.3 (SAS Institute, Cary, NC).
RESULTS
We identified 4,961 enrollees who had a claim for Parkinson’s disease and met all other study inclusion and exclusion criteria. Of these, 4,490 had a second claim with a diagnosis of Parkinson’s disease at least 30 days after the first Parkinson’s disease claim and were retained for analysis. Demographic characteristics of the Parkinson’s disease population are summarized in
Table 1 . Most of the Parkinson’s disease patients were men (61%), and more than three-fourths (77%) were 60 years old or older.
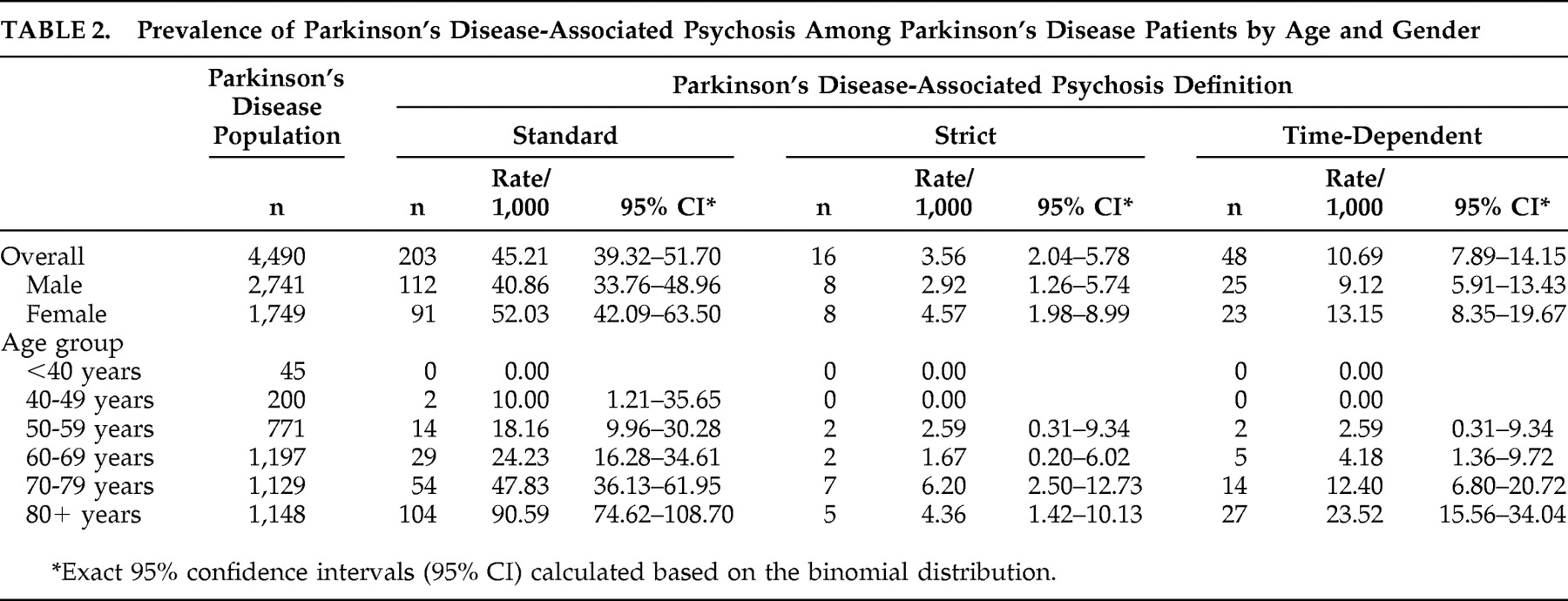
Prevalence estimates of PDP by age and gender, using the three different definitions, are reported in
Table 2 . Overall, the prevalence of PDP was estimated at 45.21/1,000 Parkinson’s disease patients using the standard definition, 3.56/1,000 using the strict definition, and 10.69/1,000 using the time-dependent definition. For all definitions, the rate of PDP among men and women was not significantly different but did increase with age (p<0.0001), with very few cases among patients younger than 50. Limiting the population to those age 50 and older, the prevalence of PDP was estimated at 47.35/1,000 Parkinson’s disease patients using the standard definition, 3.77/1,000 using the strict definition, and 11.31/1,000 using the time-dependent definition. A sensitivity analysis was conducted to provide the largest possible estimate of psychosis in the Parkinson’s disease population. Prevalence of psychosis within the identified Parkinson’s disease population before applying the exclusionary diagnostic criteria (secondary/drug-induced Parkinson’s disease, dementia with Lewy bodies, delusional disorder, or primary psychiatric disorders) was estimated at 97.4 per 1,000 Parkinson’s disease patients (95% CI=90.1–104.8).
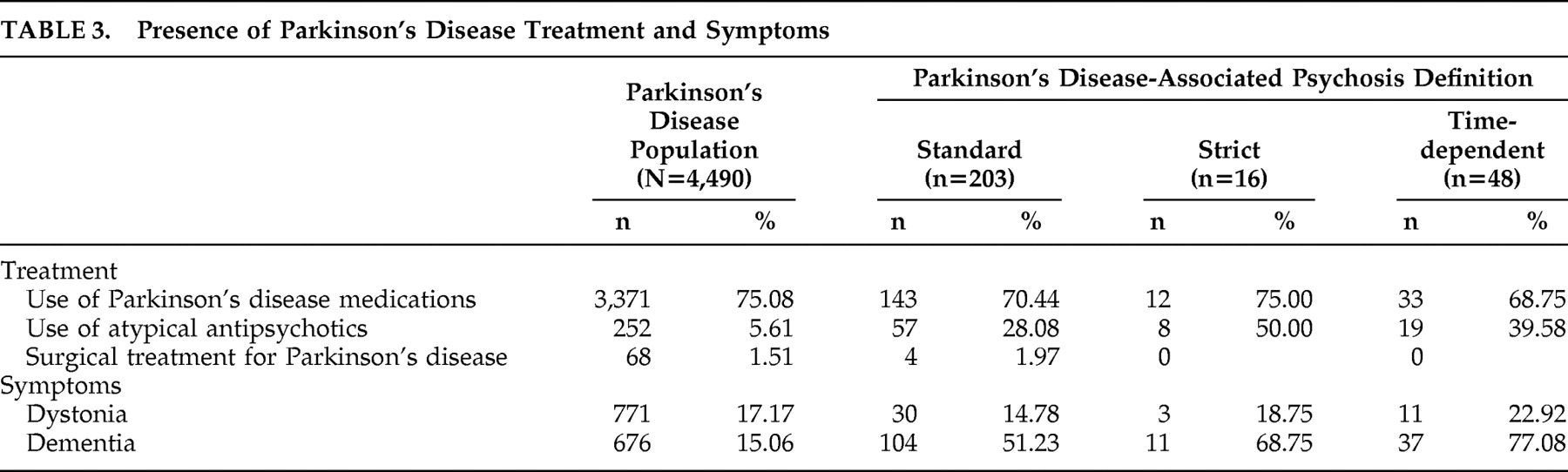
Presence of Parkinson’s disease treatments and symptoms among patients with Parkinson’s disease and PDP (using each definition) are summarized in
Table 3 . Use of Parkinson’s disease medications was consistent across all groups. Use of atypical antipsychotics in the PDP population ranged from 28% to 50%; unsurprisingly, this use was more common among patients with PDP (p<0.0001). Few patients (less than 2% of the overall Parkinson’s disease population) received surgical treatment for Parkinson’s disease. The rate of dystonia was similar in the overall Parkinson’s disease population and among patients with PDP. Dementia was present in 15% of the Parkinson’s disease population, whereas this rate was higher among PDP patients (p<0.0001), ranging from 51% to 77%.
DISCUSSION
There is limited information on the overall prevalence of Parkinson’s disease in the United States, and even less on the burden of PDP. Evidence suggests early treatment with antipsychotics may slow progression of psychotic symptoms in Parkinson’s disease and reduce the risk for later deterioration
5 ; therefore, early recognition and treatment of patients with PDP are critical. Our analysis found the prevalence of PDP in a commercial managed care population is low, ranging from 3.6/1,000 to 45.2/1,000 Parkinson’s disease patients, and a majority of these patients had no evidence of antipsychotic treatment.
An analysis of the PDP literature estimated the prevalence of PDP among patients older than 50 years old at 24% by combining the few studies available for review.
8 However, many of the reviewed studies did not provide the longitudinal detail necessary to precisely determine whether their definition of psychosis was consistent with the NINDS/NIMH working group criteria. Additionally, most Parkinson’s disease patients in those studies were recruited from specialty neurology or movement disorders clinics and were older than 50. As Parkinson’s disease is progressive and development of psychotic symptoms is more common among older individuals,
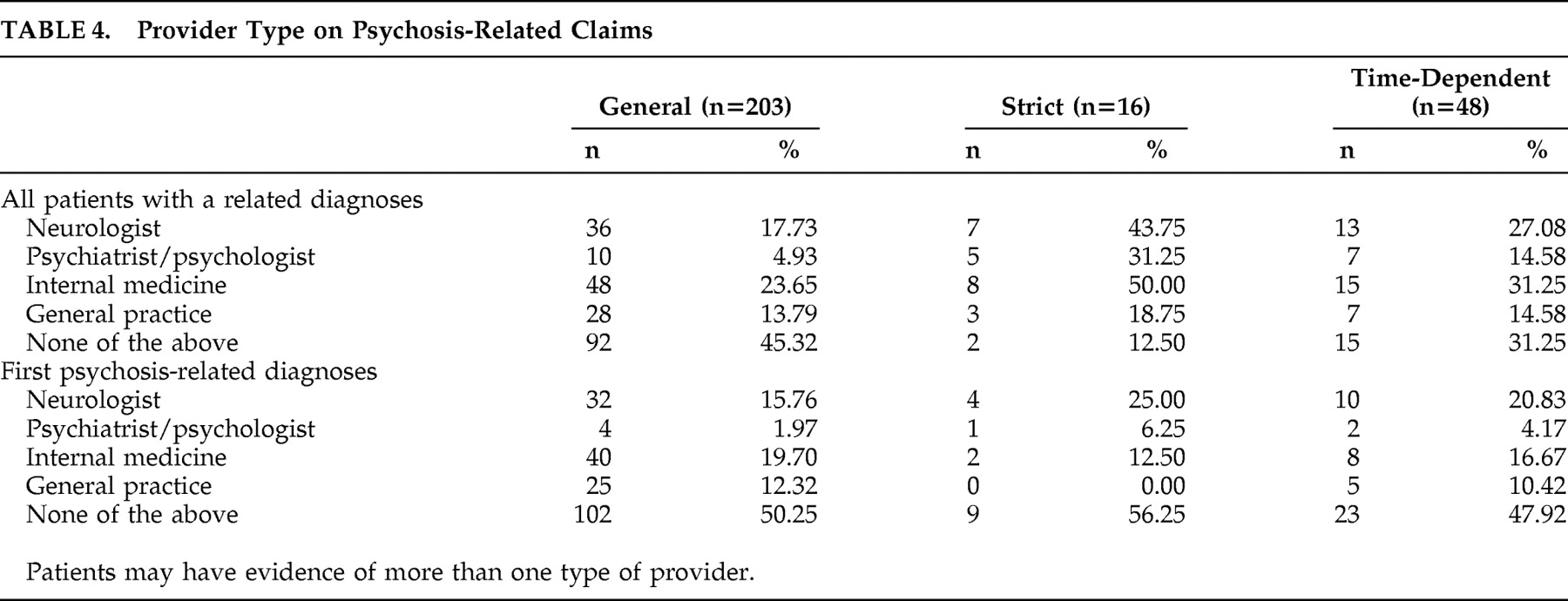
9 it is expected these patients would have more severe or progressed Parkinson’s disease and be more likely to have PDP than patients from a general Parkinson’s disease population, such as the commercial managed care population we analyzed. The data presented here appear to bear this out. Although we did not have specific data on the clinical setting from these claims data, we completed an unweighted analysis of provider specialty in each of the categorical definitions of PDP (
Table 4 ). These data show wide variability in the clinical practice specialty responsible for the diagnosis. However, in looking at a weighted frequency of the specialties, 62% of the general claims definition came from four specialty providers (neurology 14%, hospital 17%, internal medicine 19%, family medicine 12%).
We found the overall prevalence of psychosis could be as high as 10% of the Parkinson’s disease population. Previous studies of PDP that included general population-recruited Parkinson’s disease samples reported similar rates of psychotic symptoms, ranging from 6% to 14%.
10,
11 Thus, the rate estimated from our analysis is consistent with rates reported in comparable studies.
It is important to note that the prevalence estimates from our study rely on identification of specific diagnosis and symptom codes within administrative claims data and lack detailed clinical information. For example, according to the U.K. brain bank criteria, diagnosis of Parkinson’s syndrome is dependent on the presence of bradykinesia, a symptom for which there are no specific ICD-9-CM codes.
12 We addressed the lack of detailed clinical information on Parkinson’s disease symptoms by requiring patients to have at least two claims for primary/idiopathic Parkinson’s disease on separate dates for inclusion in the study population. This strategy decreases the likelihood of including patients with a single claim in error or as a rule-out condition and is consistent with other studies of chronic health conditions using claims data.
Similarly, the criteria proposed for diagnosis of PDP by the NINDS/NIMH working group are also based on clinical diagnostic measures which are not directly available in claims data. For example, the NINDS/NIMH criteria include the presence of at least one of the following as components of PDP: hallucinations, delusions, illusions, or false sense of presence. Illusions and false sense of presence cannot be elucidated from claims, as there are no ICD-9-CM diagnosis codes for them, and as such our analysis may have overlooked some patients with less severe PDP. This limitation is tempered because inclusion of these symptoms as indicators of PDP is controversial, and they are less likely to be reported spontaneously.
7 Additionally, delusions are one of the characteristic symptoms of Parkinson’s disease-associated psychosis, yet the NINDS/NIMH working group suggests exclusion of patients with a “delusional disorder.” As a solution, we excluded patients with a delusion diagnosis appearing before the identified Parkinson’s disease index date, as we felt this approach would best omit patients who may have a primary delusional disorder, and delusion diagnoses appearing on or after the study index date were assumed to be a symptom of Parkinson’s disease-associated psychosis.
Because patients with Medicare or Medicaid coverage were not analyzed in this study, our patient population may be younger, be wealthier, or have less advanced disease than Parkinson’s disease patients in the U.S. population overall, potentially limiting the generalizability of our estimates. However, the demographic characteristics of our Parkinson’s disease population were similar to those from other reports in the literature
13 –
17 ; therefore, applying our unadjusted PDP rates to the estimated U.S. Parkinson’s disease population could provide useful information about the overall burden of PDP in the United States.
Unfortunately, data about the prevalence of Parkinson’s disease in the United States are sparse and inconsistent. The Parkinson’s Disease Foundation reports that “as many as one million Americans suffer from Parkinson’s disease.”
18 However, no information is provided regarding how this number was determined. Other studies have reported vastly different estimates of the prevalence of Parkinson’s disease. One study reported an overall prevalence of Parkinson’s disease of 0.3% in the United States (approximately 930,000 Parkinson’s disease patients based on a U.S. population of 310 million people), with this rate increasing to 4%–5% in those older than 85 years.
19 However, a cross-sectional prevalence analysis in London reported an age-adjusted rate of 168/100,000, which translates to approximately 520,000 Parkinson’s disease patients in the United States.
13 A multinational study estimating the prevalence of Parkinson’s disease in the world’s most populous nations reported an estimated prevalence of 340,000 Parkinson’s disease patients at least 50 years old in the United States, with prevalence rates ranging from 128/100,000 among those younger than 65 years old to 958/100,000 among persons at least 75 years old.
20 This estimate was extrapolated from a small study in rural Mississippi,
21 and its generalizability to the United States population is questionable. Clearly, a gap in knowledge exists regarding the prevalence of Parkinson’s disease.
As the ambiguity in the prevalence of Parkinson’s disease adds to the uncertainty surrounding the true burden of PDP, we estimated the possible range of patients with PDP in the United States by multiplying our unadjusted PDP prevalence rates by the various Parkinson’s disease population sizes reported. Assuming a population of 1 million Parkinson’s disease patients in the United States,
18 we estimated that the point prevalence of PDP in the United States ranges anywhere from 3,560 to 45,210 PDP patients, with a prevalence of 10,690 PDP patients using the criteria that most closely follow the NINDS/NIMH guidelines. Using the more inclusive definition of psychosis among patients with Parkinson’s disease (i.e., without applying exclusionary diagnostic criteria), the number of Parkinson’s disease patients in the United States with psychosis may be as high as 97,400 individuals, within the range of other reported estimates.
8 Applying our unadjusted rates to the more conservative U.S. estimate of 340,000 Parkinson’s disease patients at least 50 years old,
20 we estimated the number of PDP patients in the United States ranges from 1,282 to 16,098 individuals age 50 and older, a much smaller number than previously reported. Although these estimates of PDP burden appear modest compared with other psychiatric conditions, the finding that a unique Parkinson’s disease psychosis likely exists, coupled with the intriguing evidence of early treatment efficacy, has important implications for designing treatment programs for patients with this neuropsychiatric disorder. Future research should be directed at further refinement of the definition of Parkinson’s-induced psychosis as our study indicates that the incidence can vary depending on that definition.