L ack of insight of cognitive and functional deficits is a striking symptom among many neurological patients. Different terms have been used to describe the phenomenon such as anosognosia, unawareness of deficits, and lack of insight.
1 These concepts are used synonymously in this article. In Alzheimer’s disease, impaired awareness is a common symptom even in the earliest stages,
2,
3 and the frequency of the symptom increases with disease progression.
4 Many studies have focused on the association between impaired awareness in Alzheimer’s disease and different cognitive functions and the neurobiological substrate for awareness.
5 Fewer studies have investigated if impaired insight is related to neuropsychiatric symptoms. The majority of studies that have investigated the relationship between psychiatric symptoms and awareness have studied the association between insight and depression. In a recent review of the clinical correlates for awareness in dementia, it was concluded that dysthymia, but not major depression, may be related to higher levels of awareness.
6 Impaired awareness may also be related to increased apathy,
4,
7 –
9 agitation,
10 irritability
11 and psychosis.
4,
12,
13 The majority of patients included in these studies had moderate to severe dementia, and less is known about the relation between neuropsychiatric symptoms and insight in early Alzheimer’s disease.
The objective of this study was to investigate whether impaired awareness of deficits was associated with higher frequency of neuropsychiatric symptoms in a large sample of patients with early Alzheimer’s disease. Further, we wished to assess the potential distress associated with neuropsychiatric symptoms in caregivers of patients with impaired awareness.
METHODS
This study was based on baseline data from an ongoing Danish longitudinal multicenter study, the Danish Alzheimer Intervention Study (DAISY), which investigates the efficacy of an intensive structured intervention program with counseling and support to patients with early phase Alzheimer’s disease and their caregivers.
DAISY was performed in five Danish regions. It was designed as a multicenter single-blind, randomized, controlled trial with randomization to an intensive program or to usual care and 1 year follow-up. Data in this study was from the baseline examination. At baseline all patients and their primary caregiver were interviewed using separate structured questionnaires. The interviews were performed by the local project coordinator in each of the five centers.
Participants
The study included 330 home-living patients with a recent clinical diagnosis (within less than 12 months of inclusion) of probable Alzheimer’s disease, mixed Alzheimer’s disease (defined as patients meeting the criteria for probable Alzheimer’s disease with minor vascular lesions, which could not explain the cognitive impairment), or dementia with Lewy bodies confirmed by the local specialist referral unit (memory clinic). In this study focusing on Alzheimer’s disease, patients with dementia with Lewy bodies (n=9) were excluded. Thus, the study population consisted of 321 patients who met the NINCDS-ADRDA criteria for probable Alzheimer’s disease.
14 Further inclusion criteria were age at least 50 years old and Mini-Mental State Examination (MMSE) score of 20 or above. Further, patients needed a primary caregiver with at least once per week contact who was willing to participate in the study. Exclusion criteria were severe somatic or psychiatric comorbidity (including impaired hearing or vision), which would significantly impair cooperation in the program. Patients with severe psychiatric comorbidity were excluded because participants should be able to follow a counseling program, which was the primary aim of the DAISY. The number of excluded patients with psychiatric comorbidity has not been recorded, but it was fewer than 10. Patients participating in other intervention studies at inclusion or during the study were excluded. Patients were recruited from local memory clinics, local specialists in psychiatry, neurology, and geriatrics, as well as from general practitioners.
The DAISY project was approved by Danish Data Protection Agency (j. nr. 2003–41-3178), by the Ethical Committee (j. nr. (KF) 02–005/04), and the trial was registered in Current Controlled Trials number ISRCTN74848736 (http://controlled-trials.com). All patients and caregivers gave informed consent to participation in the study.
Assessment of Awareness
Anosognosia Rating Scale
The categorical three point scale from Reed et al.
15 was used. This scale has high interrater reliability
15 and corresponds well with other measures of anosognosia.
3 Based on cognitive testing and interviews with the patient and the caregiver, a clinician rated the patient’s level of awareness. Awareness was classified into the following categories: “full awareness,” “shallow awareness,” and “no awareness.” For a detailed description, see Reed et al.
15Memory Discrepancy Rating
Patients were asked to rate their memory on a 5-point scale with the following classification: “very good,” “good,” “fair,” “poor,” “very poor.” Caregivers were also asked to rate the patients’ memory abilities using the same scale. The difference between the caregivers’ and the patients’ score was used as a measure for awareness (possible score range of 1–5; higher [positive] discrepancy scores indicated that the caregiver rated the patients worse than the patients rated themselves).
Neuropsychiatric Inventory-Questionnaire (NPI-Q) 16
The NPI-Q measures 12 categories of behavioral disturbance: delusions, hallucinations, anxiety, depression/dysphoria, agitation/aggression, elation/euphoria, disinhibition, irritability/lability, apathy/indifference, motor disturbance, nighttime behavior problems, and problems with appetite/eating. The NPI-Q was administered to the caregiver in interview form. The caregiver was first asked if the symptoms occurred, and if they responded yes, the caregiver then was asked to rate the severity of the behavior exhibited on a scale of 1 to 3, with 3 being the most severe. The NPI-Q yields a total severity score for the patient, which is the sum of the severity scores obtained for each behavioral category (labeled NPI-Q total severity score in this manuscript). Additionally, the caregiver was asked to rank their level of distress from each behavior, on a scale of 1 to 5, with 5 indicating the most severe level of distress. The NPI-Q yields a total distress score, which is the sum of the distress scores obtained for each behavioral category. This score is labeled NPI-Q total distress score in this manuscript.
Cornell Scale for Depression in Dementia 17
The Cornell Scale for Depression in Dementia was used to measure mood disturbances in patients. The clinician-administered scale uses information from interviews with both patients and their caregivers. The scale contains 19 items with item scores ranging from 0 (absent) to 2 (severe). The maximum score is 38 points.
Statistical Analysis
The significance of differences between the three groups as divided by the Anosognosia Rating Scale was assessed by one way analysis of variance (ANOVA). The Kruskal-Wallis test was used when variances in homogeneity of variances were found. For continuous variables, independent sample t tests were applied to test the significance of differences between patient groups. The Mann-Whitney U test was used to test the significance of differences between variables where equal variances were not found. Bonferroni’s correction was used when multiple comparisons were performed. The significance of correlations for the Memory Discrepancy Rating score with other variables was investigated with Spearman correlations. The level of significance was set at p≤0.05.
RESULTS
Patient and Caregiver Characteristics and Insight Ratings
Of the 321 included patients, 145 were men (45%) and 176 were women (55%). The mean age of the patients was 76.2±7.2 years (range=54–92 years). Patients’ mean MMSE score was 24.04±2.59. Caregivers’ mean age was 66.2±12.7 years (range=22–90 years), and 211 of them were spouses (66%). The remaining caregivers were children (26%) or were in some other way related to the patient (8%). There were no paid caregivers. Patients lived with caregivers in 211 cases (66%) (two children lived with their parent), and 95% of the patients had contact daily or several times a week with their caregiver.
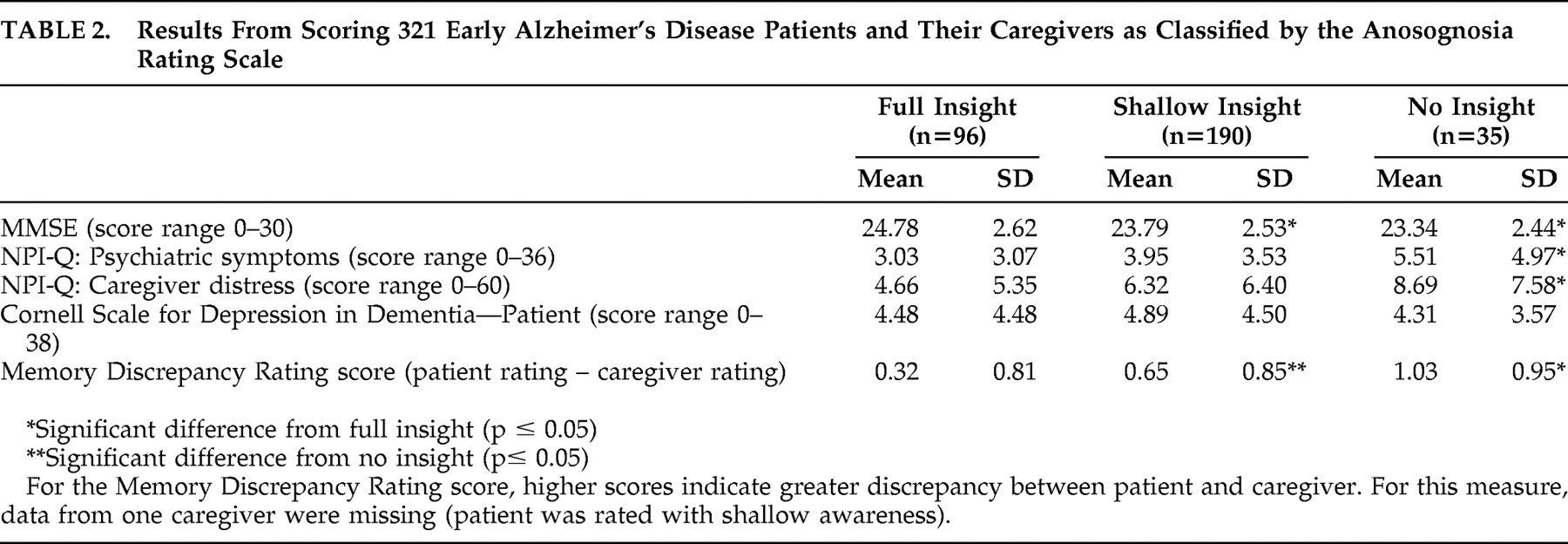
On the Anosognosia Rating Scale, 96 patients (30%) had “full insight,” 190 patients (59%) had “shallow insight,” and 35 patients (11%) were rated with “no insight.” Results from the memory rating score are shown in
Table 1 . In the patient group, 182 patients (57%) rated their memory better than their caregiver, and 105 patients and caregivers (33%) gave the same ratings. In 33 cases (10%) caregivers rated the patients’ memory better than the patients.
Neuropsychiatric Inventory-Questionnaire (NPI-Q) and Depression Rating
Patients’ mean NPI-Q total severity score was 3.84±3.6 (range=0–18). The mean NPI-Q total distress score was 6.08±6.3 (range=0–33). The mean Cornell Scale for Depression in Dementia score was 4.7±4.3.
Caregivers indicated that a given neuropsychiatric symptom occurred with the following frequency: 15.9% delusions, 12.1% hallucinations, 28.7% anxiety, 36.1% depression/dysphoria, 23.4% agitation/aggression, 8.1% elation/euphoria, 16.1% disinhibition, 42.9% irritability/lability, 38.6% apathy/indifference, 16.5% motor disturbance, 10.9% nighttime behavior problems, and 21.2% experienced problems with appetite/eating. Thus, the three most frequent neuropsychiatric symptoms were irritability, apathy, and depression. Fifty-two patients (16.2%) had no positive score on any neuropsychiatric symptom.
Relation Between Insight and Neuropsychiatric Symptoms and Dementia Severity
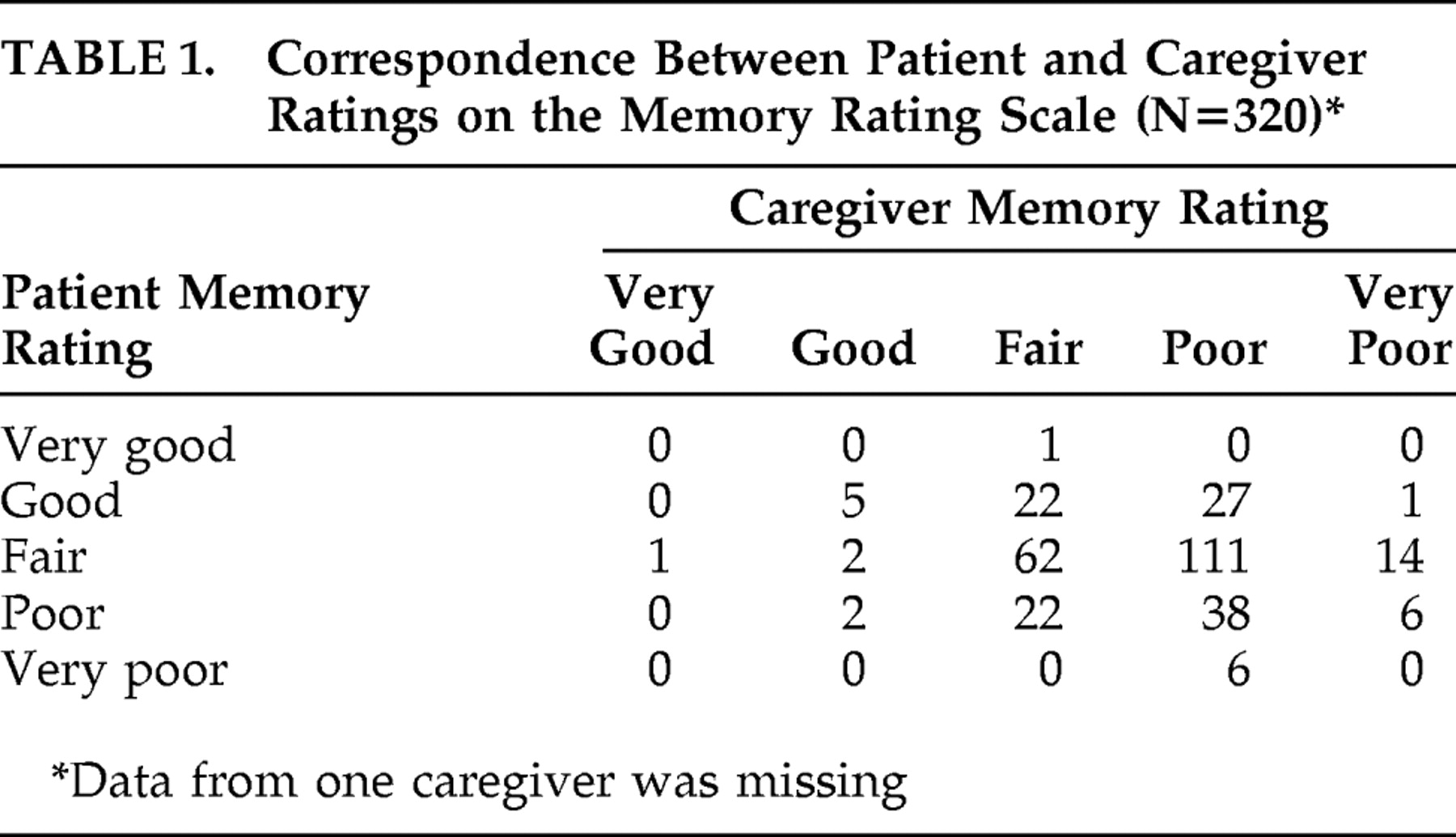
In
Table 2, NPI-Q total severity score, NPI-Q total distress, and patients’ depression score (Cornell Scale for Depression in Dementia) are displayed for the three patient groups as classified by the Anosognosia Rating Scale. A significant group effect was found for NPI-Q total severity score (χ
2 =8.4, df=2, p=0.015) and for MMSE score (F=6.2, df=2, 318, p=0.002), but not for Cornell Scale for Depression in Dementia (F=0.43, df=2, 318, p=0.65). Post hoc t tests (with Bonferroni’s correction) showed that patients with full and no insight differed significantly with respect to MMSE score. Further, the MMSE score was significantly higher in patients with full insight relative to no insight. Post hoc test with the Mann-Whitney U test showed that the NPI-Q total severity score was only significantly higher in patients with no insight relative to patients with full insight. Since significant group effects of insight classification were found for both MMSE and NPI-Q total severity scores we controlled the group differences in NPI-Q total severity score for the influence of dementia severity (MMSE). In the analysis of covariance the effect of insight classification in the NPI-Q total severity score remained significant (F=4.25, p=0.006). Subanalyses were performed to examine the impact of living status and the type of relation between patient and caregiver on NPI-Q total severity score and the Memory Discrepancy Rating score. No significant results were found.
The Memory Discrepancy Rating score was significantly correlated to NPI-Q total severity score (r=0.19, p=0.001) but not to Cornell Scale for Depression in Dementia (r=−0.07, p=0.22). Thus, insight had a significant impact on the frequency of neuropsychiatric symptoms (independent of dementia severity), but it was not related to depressive symptoms.
Correspondence Between Insight Measures
The correspondence of the Anosognosia Rating Scale and the Memory Discrepancy Rating score was assessed by analyzing the scores from the Memory Discrepancy Rating score classified by ratings on the Anosognosia Rating Scale. Results are presented in
Table 1 . For the difference measure (patient rating − caregiver rating) a significant group effect was found (F=9.8, df=2, 317, p<0.001). All three groups as classified by the Anosognosia Rating Scale differed significantly with post hoc t tests with Bonferroni correction. Thus, the Memory Discrepancy Rating score corresponded well with the classification by clinician.
Association Between Impaired Awareness and Caregiver Distress
The results from NPI-Q total distress score are also shown in
Table 1 . A significant group effect was found for NPI-Q total distress score (χ
2 =9.99, df=2, p=0.007), and post hoc tests with the Mann-Whitney U test showed that NPI-Q total distress score was only significantly higher in patients with no insight as compared to patients with full insight. The Memory Discrepancy Rating score was significantly correlated to NPI-Q total distress score (r=0.22, p<0.001). Living status and the type of relation between patient and caregiver were not significantly related to NPI-Q total distress score.
Associations Between Neuropsychiatric Symptoms, Caregiver Distress, and Dementia Severity
A significant high positive correlation was found between NPI-Q total severity score and NPI-Q total distress score (r=0.89, p<0.001). MMSE was not significantly correlated with NPI-Q total severity score (r=−0.018, p=0.75) or NPI-Q total distress score (r=0.002, p=0.97). Thus, caregivers who reported more neuropsychiatric symptoms in patients experienced these as distressful. Dementia severity did not have a significant impact on neuropsychiatric symptoms in this patient sample.
DISCUSSION
In accordance with many previous studies, this study showed that impaired awareness of deficits occurs in the majority of patients with early Alzheimer’s disease.
2,
3 Importantly, the study demonstrated that early Alzheimer’s disease patients with very poor insight have significantly more neuropsychiatric symptoms than patients who are fully aware of their cognitive deficits. This study focused on neuropsychiatric symptoms in general, but since the most frequent neuropsychiatric symptoms were irritability, apathy, depression, anxiety, and agitation, the results primarily reflect these symptoms. The study showed that although these symptoms occur frequently in early Alzheimer’s disease, the severity is mild. The prevalence of different neuropsychiatric symptoms in our study resembles previous results.
18Previous studies have also found that impaired awareness may be related to different neuropsychiatric symptoms including increased apathy,
4,
7 –
9 agitation,
10 irritability,
11 psychosis
4,
12,
13 or behavioral symptoms in general.
5 Previous studies did not focus specifically on early Alzheimer’s disease patients but included also patients with moderate or severe dementia. Thus, our study not only corroborated the findings from previous studies but (in a large patient group) demonstrated that impaired awareness may be an important factor for the occurrence of neuropsychiatric symptoms—even in the early phases of Alzheimer’s disease.
Most studies on psychiatric symptoms and impaired awareness have investigated the relationship between impaired awareness and depression. The majority of studies have found that dysthymia, but not major depression, is probably related to higher levels of awareness, although some inconsistencies exist probably due to methodological differences (for review see Aalten et al.
6 ). It has been suggested that dysthymia may occur in patients with higher levels of awareness as an emotional response to cognitive decline,
2 and this explanation seems logical for many patients.
Our study did not find a significant association between the degree of insight and depressive symptoms, which is in accordance with some previous studies.
7,
15,
19 That our study failed to find depressive symptoms in patients with higher levels of insight might result from the fact that the patients had recently been diagnosed and that they were very early in the disease process. Further, patients participated in a psychosocial intervention study which may have caused selection bias. The selection bias may have been in different directions. One possibility is that depressive patients are less likely to participate in psychosocial studies, leading to an underestimating of depression in patients with full insight. Another possibility is that patients with severely impaired awareness chose not to participate in the intervention study. This may be the case in our study since the proportion of patients with no insight was smaller as compared to a previous study (with consecutive patients) where the same categorical insight scale was administered.
3 In general, our results may be biased because patients were about to participate in an intervention study. Thus patients with few resources (e.g., social) were probably less likely than others to participate in the study. Further, all patients had caregivers with sufficient resources for participation. This may also have biased the participants toward the mild end of the disease spectrum.
The relationship between neuropsychiatric symptoms and impaired insight may result from a common neurobiological basis. Some previous studies have found that both symptoms may occur from right hemisphere dysfunction (especially frontal regions). Both impaired awareness and apathy have been found to correlate significantly with right hemisphere dysfunction in Alzheimer’s disease
8 as well as in Parkinson’s disease.
20 Psychosis has also been related to right frontal cortical structures (and related subcortical structures).
21This study showed a highly significant association between neuropsychiatric symptoms and caregiver distress. Unfortunately, the data presented here cannot be used to investigate if impaired awareness is directly associated with higher caregiver distress, because distress questions in NPI-Q are asked in relation to neuropsychiatric symptoms and not to distress in general. However, results from previous studies have indicated that impaired awareness may be directly related to higher caregiver burden.
11,
22,
23 In accordance with these studies our findings showed that when patients had increasing neuropsychiatric symptoms, caregivers reported higher levels of distress.
To summarize, our study showed that impaired awareness of deficits occurs frequently in early Alzheimer’s disease. We also found that lower levels of awareness were significantly associated with the occurrence of neuropsychiatric symptoms—even in early Alzheimer’s disease. Specifically, patients with very poor insight have more neuropsychiatric symptoms. Thus, when evaluating the need of patients and caregivers in early Alzheimer’s disease, identification of impaired insight may be important.
Acknowledgments
The authors would like to thank 128 volunteering raters and assistants from 68 municipalities and five counties who have contributed to DAISY.
The following hospitals took part in the DAISY study: Ribe County: Esbjerg Hospital, Department of Psychiatry (Anna Marie Hansen, Johanne Christensen, Jørgen Jensen); Ringkoebing County: Herning Hospital, Department of Psychiatry (Marianne Refslund, Birgitte Aagaard, Palle Lund), and Holstebro Hospital, Department of Psychiatry (Inge Lund Petersen, Finn Andersen); Roskilde County: Roskilde Hospital, Department of Geriatrics (Dorte Dyre, Lisbeth Petersen, Birgitte Frølund, Lise Korbo, Ellen Holm) and Department of Neurology (Kurt Lüdorff); Vestsjaelland County: Korsoer Hospital, Department of Geriatrics (Mette Lassen, Lars Laugesen), and Dianalund Hospital, Department of Psychiatry (Thye Jensen, Ole Bjørn Skausig); Copenhagen, Denmark, Capital area: Hvidovre Hospital, Department of Geriatrics (Lillian Moerck Joergensen); Amager Hospital, Department of Geriatrics (Suzanne Sanders); Bispebjerg Hospital, Department of Geriatrics (Claus Moe); Frederiksberg Community Health Care Center (Ingrid Lauridsen); Frederiksberg Hospital, Department of Psychiatry (Rene Klysner); Glostrup Hospital, Department of Neurology, (Jens Feilberg); Rigshospitalet, Department of Neurology (Ane Eckerman, Eva Illemann, Susanne Rishoej, Lisbeth Villemoes Soerensen, Peter Johannsen).
The Danish Alzheimer Intervention Study was supported by the National Board of Social Services, the Ministry of Social Affairs, with contributions from the Ministry of Health, the Danish Health Foundation, and the Danish Alzheimer Research Foundation.